Advanced Maternal Age and Nicotine Consumption during Pregnancy: Additive
Effects on Newborn Parameters
Sylvia Kirchengast ✉
✉
University of Vienna, Department of Evolutionary Anthropology, Althanstrasse
14, A-1090 Vienna, Austria.
University of Vienna, Department of Evolutionary Anthropology, Althanstrasse
14, A-1090 Vienna, Austria.
University of Vienna, Department of Evolutionary Anthropology, Althanstrasse
14, A-1090 Vienna, Austria.
University of Vienna, Department of Evolutionary Anthropology, Althanstrasse
14, A-1090 Vienna, Austria.
Medical University of Vienna, Department of Obstetrics and Gynecology.
DOI: https://doi.org/10.52905/hbph.v1.6
Abstract
Background
Nicotine consumption during pregnancy and advanced maternal age are well known
independent risk factors for poor pregnancy outcome and therefore serious public health
problems.
Objectives
Considering the ongoing trend of delaying childbirth in our society, this study
investigates potential additive effects of nicotine consumption during pregnancy and
advanced maternal age on foetal growth.
Sample and Methods
In a medical record-based study, we analysed the impact of maternal age and smoking
behaviour before and during pregnancy on newborn size among 4142 singleton births that
took place in Vienna, Austria between 1990 and 1995.
Results
Birth weight (H=82.176, p<0.001), birth length
(H=91.525, p<0.001) and head circumference
(H=42.097, p<0.001) differed significantly
according to maternal smoking behaviour. For birth weight, the adjusted mean differences
between smokers and non-smokers increased from 101.8g for the < 18-year-old mothers
to 254.8g for >35 year olds, with the respective values for birth length being 0.6 cm
to 0.7cm, for head circumference from 0.3 cm to 0.6 cm.
Conclusion
Increasing maternal age amplified the negative effects of smoking during pregnancy on
newborn parameters. Our findings identify older smoking mothers as a high-risk group
which should be of special interest for public health systems.
Keywords: advanced maternal age at first birth, maternal nicotine consumption, foetal growth, newborn size, birth weight, smoking
Conflict of Interest: There are no
conflicts of interest.
Citation: Koger, R et al. (2021), Advanced Maternal Age and Nicotine Consumption during Pregnancy: Additive
Effects on Newborn Parameters, Human Biology and Public Health 1. https://doi.org/10.52905/hbph.v1.6.
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 09-11-2020 | Accepted: 30-11-2020 | Published: 22-06-2021
Take home message
Nicotine consumption during pregnancy and advanced maternal age have an additive negative
effect on foetal growth. Smoking pregnant women older than 35 years are a high-risk group
which is of special importance for public health programmes.
Contents
Introduction
For more than four decades, most high-income countries have been confronted with a
remarkable demographic change in childbearing patterns. Starting in the early 1960s the
trend has been to delay first childbirth (Wilkie
1981; Huang et al. 2008). This trend was
caused by the introduction of effective contraceptives, marked changes in female role
models, such as the women’s liberation movement, prolonged phases of education and newly
defined career goals. Consequently, pregnancy at advanced maternal age has become
increasingly common, primarily in developed but also in some developing countries (Huang et al. 2008). In most high-income countries, the
proportion of women who gave first birth at the age of 35 years or older increased
significantly. In the United States, this proportion increased nearly eight times between
1970 and 2006 (Kenny et al. 2013). Similar trends
are reported for Sweden (Jacobsson et al. 2004),
the United Kingdom (Fitzpatrick et al. 2017),
Poland (Radoń-Pokracka et al. 2019), Japan (Ogawa et al. 2017), China (Shan et al. 2018) and many other developed countries. Between 1970 and
2000, the mean maternal age at first birth increased from 24.4 to 28.5 years in Sweden, from
21.4 to 24.9 years in the United States and from 25.6 to 28.0 years in Japan (Jacobsson et al. 2004). In Austria, the average
maternal age at first birth increased from 23.8 years in 1984 to 29.9 in 2019 (Statistik Austria 2019). Currently, delaying
reproduction and giving birth at an advanced age is a worldwide trend. But what does
“advanced maternal age” actually mean? Traditionally, women 35 years old and older were
considered as elderly gravidas (Dulitzki 1998).
Today, however, advanced maternal age is defined as 40 years and, the term “very advanced
maternal age” is applied to women who are at least 45 years old (Kahveci et al. 2018).
This trend of postponing motherhood, however, also poses certain risks. It is a
well-established fact that delaying childbirth beyond the age of 35 or 40 years is not only
associated with reduced fertility, but also with several adverse obstetric outcomes. These
include increased rates of abortion, stillbirths, preterm births, low birth weight,
intrauterine growth restriction unexplained foetal death and even increased rates of
Caesarean section (Jolly et al. 2000; Hoffman et al. 2007; Aliyu et al. 2008). Moreover, complications during pregnancy are more frequent in
older than in younger women, which results in higher costs for the health system (Tromp et al. 2011). Consequently, there is a clear
effect of maternal age on foetal development and birth outcomes (Cleary-Goldman et al. 2005; Briggs
et al. 2007; Salem Yaniv et al. 2011).
This calls for identifying additive effects of advanced maternal age and other stress
factors that have a negative impact on pregnancy, intrauterine development and birth
outcome.
A well-documented stress factor during pregnancy is nicotine consumption. The negative
effects of smoking during pregnancy on foetal development are undeniable. Many studies have
shown negative influences on foetal growth and development (Voigt et al. 2009; Prabhu et al.
2010; Ekblad et al. 2015) . More
specifically, birthweight and birth length are drastically affected by nicotine consumption
during pregnancy. This activity is associated with smaller and lighter babies (Kirchengast and Hartmann 2003).
Kharkova and Odland (2019) showed that smoking during pregnancy has a negative effect on the
head circumferences of newborns. If, however, the women stopped smoking in the first
trimester of pregnancy, the head circumference was similar to that of children of
non-smokers. Mook-Kanamori et al. (2010) reported that smoking throughout pregnancy is
associated with a shorter crown to rump length in the first trimester. Furthermore, Jaddoe et al. (2007b) showed that smoking during pregnancy leads to a reduced growth of foetal
femur length, head circumference and abdominal circumference. Maternal smoking in late
pregnancy increases the risk for low birthweight and preterm birth, moreover, passive
smoking also negatively impacts birth weight (Jaddoe
et al. 2008). Importantly, smoking during pregnancy not only influences newborn
parameters, such as birth length and weight, it is also associated with negative and adverse
events during birth, such as an increased Caesarean section rate (Kirchengast and Hartmann 2003). The long-term consequences of nicotine
consumption during pregnancy are indisputable as well, with negative effects observable well
into adolescence (Knopik et al. 2012; Toledo-Rodriguez et al. 2010).
A wealth of scientific evidence demonstrates that advanced maternal age as well as nicotine
consumption during pregnancy impact foetal development. At the same time, however, little is
known about the additive effects of these two factors on child development. Some evidence
suggests that increasing age of smokers aggravates the negative effects on the foetal
development and newborn parameters (Cnattingius et al.
1985; Cnattingius 1990; Cnattingius 1997). Furthermore, Lamminpää et al. (2013) showed
that smoking and maternal age over 35 years are additive risks on adverse birth outcomes
such as preterm birth, small size for gestational age, low birth weight and foetal
death.
The present study investigates the interaction of maternal age and nicotine consumption on
newborn size as well as several vital parameters. Our hypothesis is that increasing maternal
age amplifies the negative effects of smoking during pregnancy on the newborn
parameters.
Sample and Methods
This medical record- based study analysed a data set of 4142 singleton births at the
University Clinic for Gynaecology and Obstetrics in Vienna between 1990 and 1995. This
clinic is one of the largest birth clinics in Austria with about 2500 births every year.
Prenatal check-ups are also performed there. In the present study, only births, that
fulfilled the following very strict inclusion criteria such as singleton term birth
(39th and 40th gestational week) of healthy nulliparous mothers of
Austrian or Central European origin. Healthy was defined as no registered maternal diseases
before and during pregnancy, no hypertension (BP < 150/90 mmHg), no protein or glucose in
the urine, no pregnancy related immunization, the absence of HIV infections and gestational
diabetes, and no alcohol abuse, or praeclampisa. All prenatal check-ups of the Austrian
mother-child passport had to be completed. Additional strict exclusion criteria were any
type of medically assisted reproduction such as IVF and congenital maldeformations of the
foetus.
Gestational age was calculated in terms of the number of weeks from the beginning of the
last menstrual bleeding to the date of delivery (= duration of amenorrhoea) and by two
consecutive ultrasound examinations performed before the 12th week of
gestation.
The mean age of mothers was 25.2 (± 5.6) years, with the youngest mother being 13 years old
and the oldest 46 years old. The mothers were divided into three age groups, whereby the
women between the age of 18 and 35 were put into one group associated with the “ideal age
for pregnancy”. Mothers younger than 18 years were defined as young mothers, whereas a
maternal age above 35 years was defined as advanced maternal age.
Maternal somatometric parameters
The following maternal somatometric parameters were determined according to the
recommendations of Knußmann (1988) at the first prenatal visit: Body height and
pre-pregnancy weight. Height was measured to the nearest 0.5cm using a standard
anthropometer. Pre-pregnancy weight was obtained by interview using the retrospective
method. Additionally, body weight was measured to the nearest 0.1 kg on a balance beam
scale. According to Gueri et al. (1982), gestational weight gain is extremely low
during the first 13 weeks of gestation. Consequently, pre-pregnancy weight was calculated
as the mean value of the reported weight and the weight determined at the 8th
week of gestation. Finally, maternal weight was measured before delivery (= at the end of
pregnancy). The weight gain during pregnancy was calculated by subtracting pre-pregnancy
weight from the body weight before delivery. Maternal pre-pregnancy weight status was
determined by means of the body mass index (BMI) kg/m2. To classify maternal
weight status, the cut-offs published by the WHO (2000) were used: underweight = BMI <
18.50 kg/m2; normal weight = BMI 18.50 kg/m2 to 24.99
kg/m2; overweight = BMI 25.00 kg/m2 to 29.99 kg/m2;
obese = BMI > 30.00 kg/m2.
Nicotine consumption
Nicotine consumption was documented during prenatal check-ups at the University Clinic
and subsequently categorised as follows: non-smokers, 1-5 cigarettes daily, 6-10
cigarettes daily, 11-20 cigarettes daily and more than 20 cigarettes daily. Changes in
smoking behaviour during pregnancy were also documented and divided into 4 subgroups:
non-smokers before and during pregnancy (0), non-smokers only during pregnancy (1),
smokers before and during pregnancy (2), and smokers only during pregnancy (3).
Newborn parameters
Newborn measurements were taken immediately after birth, including birth length (cm),
birth weight (g), and head circumference (cm). Birth weight is measured with a newborn
scale, birth length with an infant-meter from head to heel and the head circumference with
a measuring tape.
To evaluate the newborn vital functions, the one- and five-minute APGAR scores were used.
The APGAR score was introduced in 1952 as a simple and repeatable method to assess the
health status of newborns immediately after birth. Five simple criteria – skin
color/complexion, pulse rate, reflex irritability, muscle tone and breathing – are
evaluated on a scale from zero to ten. The APGAR scoring system remains as relevant for
predicting neonatal survival today as it was 60 years ago (Casey et al. 2001).
Statistical analysis
For statistical analyses SPSS for Windows (version 26.00) was used. After computing
descriptive statistics including the Kolmogoroff Smirnov test, group differences in
maternal and newborn parameters between the four smoking categories were tested by the
Kruskal-Wallis H-test with Bonferroni corrections. Pearson
χ2 tests were used to test differences
between age categories and maternal smoking behavior. Multiple regression models were
calculated including birth weight, birth length and head circumference as dependent
variables, independent variables were maternal age, nicotine consumption during pregnancy,
BMI, maternal body height and weight gain during pregnancy. For calculating adjusted mean
differences for newborn parameters between smokers and non-smokers in each age category
linear regression models were used p<0.05 was considered as
statistically significant.
Results
Sample description
Table 1 summarises maternal and newborn
characteristics. Most mothers were classified as normal weight, 15.5% of the mothers were
overweight, only 4.4% corresponded to the definition of obesity. The proportions of
smokers and non-smokers before pregnancy were 36.1% and 63.9% respectively, changing to
28.6% and 71.4% of smokers and non-smokers during pregnancy.
Smoking behaviour during pregnancy
The analysis of the maternal smoking behaviour shows that most of the women reduced or
stopped smoking with pregnancy (Figure 1).
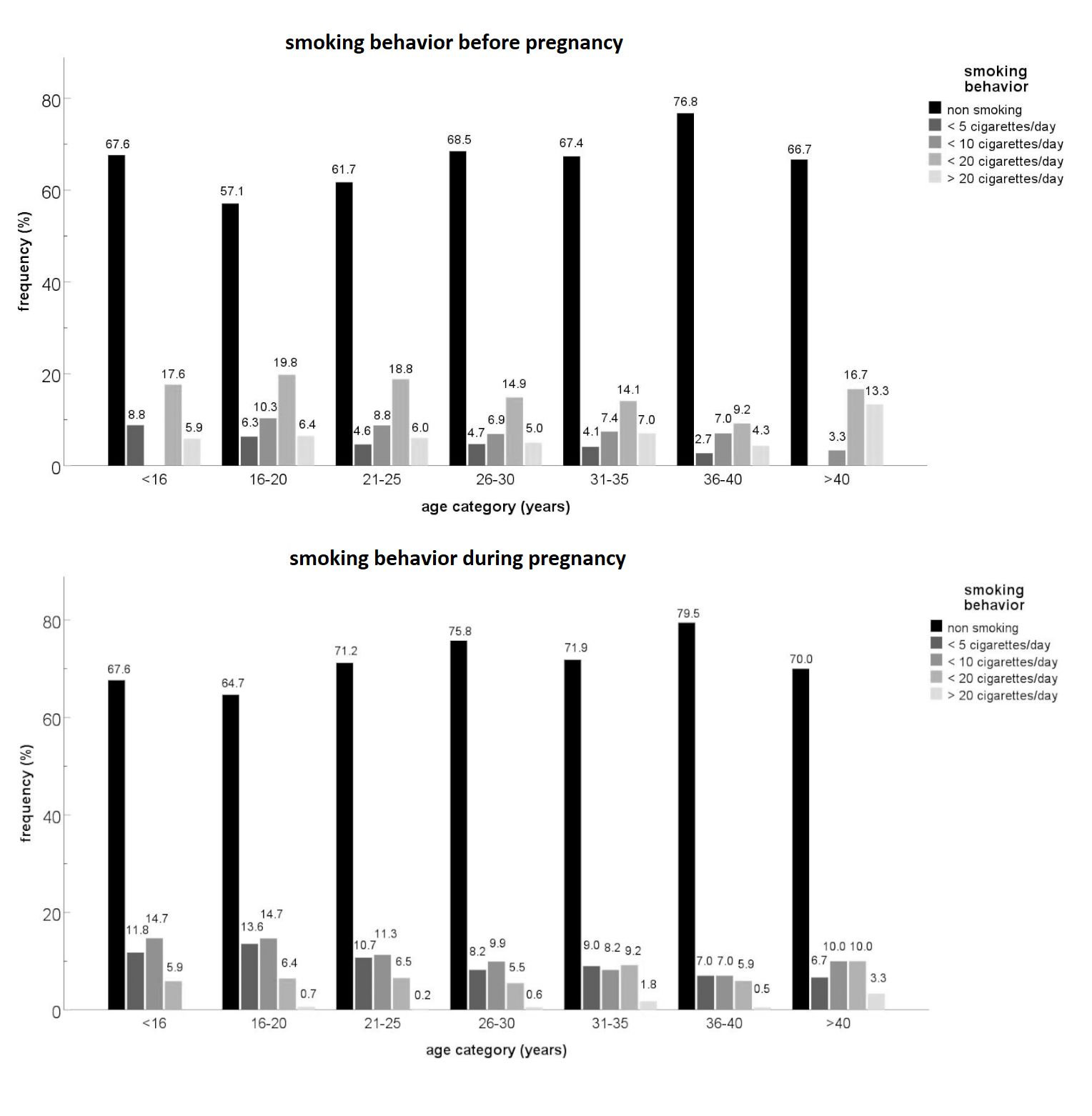
Figure 1 Comparison between the amount of daily smoked cigarettes before and during
pregnancy
The highest age category (>40 years) encompassed the most women who smoked more than
twenty cigarettes a day during pregnancy. As evident in Table 2, smoking behaviour
differed significantly between the maternal age groups
(χ²=25.411, df=6,
p<0.001). More younger women (<18 years) continued smoking (31.1%)
or even started smoking during pregnancy (7.8%), than women of 18 to 35 years of age
(23.7% and 4.8% respectively) and the oldest mothers (17.2% and 4.7% respectively). In the
oldest age category (>35 years) the proportion of non-smokers (70.7%) was higher than
expected but fewer women stopped smoking than expected (7.4%).
Smoking behaviour during pregnancy and maternal as well as newborn parameters
Birth weight (H=82.176, p<0.001), birth length
(H=91.525, p<0.001) and head circumference
(H=42.097, p<0.001) differed significantly between
the smoking categories (Table 3). The post-hoc
tests show that the offspring of absolute non-smokers and of the smokers who quit during
pregnancy were significantly heavier than smokers before and during pregnancy. The latter
group had significantly shorter offsprings than the absolute non-smokers and the
non-smokers only during pregnancy. Additionally, the offspring of the absolute non-smokers
are significantly larger than those of the smokers only during pregnancy. The head
circumferences of the newborns of the absolute non-smokers are significantly larger than
those of the smokers before and during pregnancy. The APGAR 1 and APGAR 5 scores did not
differ significantly between the groups.
To analyse the independent effect of nicotine consumption and age on the newborn
parameters (birth weight, birth length, head circumference) we performed a multiple
regression model (Table 4). The effect was
corrected for the following parameters: the height of the mother, her weight gain during
pregnancy and her pre-pregnancy BMI. The results show that smoking behaviour and maternal
age had an independent effect on all three parameters: birth weight
(R²=0.148, p<0.001), birth length
(R²=0.114, p<0.001) and head circumference
(R²=0.079, p<0.001). Smoking during pregnancy had a
negative effect on those parameters, while the maternal age had a positive effect. With
increasing maternal age, the newborns became bigger and heavier. Newborns of smokers were
in general smaller and lighter, independent of the maternal age.
Smoking behaviour and maternal age
Kruskal-Wallis H-tests and Dunn-Bonferroni post-hoc tests were performed
to test differences in maternal (Table 5) and
newborn parameters (Table 6) between age
categories in each smoking behaviour category.
Maternal stature (H=13.353, p<0.05), pre-pregnancy
BMI (H=31.658, p<0.05), birth weight
(H=13.160, p<0.05) and head circumference
(H=18.689, p<0.05) differed significantly between
the maternal age groups among the non-smoking before and during pregnancy category. Those
under 18 years and the 18-35-year-olds differed significantly in stature, BMI, birth
weight and head circumference, in the sense that the values of these parameters of the
<18-year-olds are smaller. Additionally, the <18 years have a significantly lower
BMI, birth weight and head circumference than the >35-year-olds. Concerning head
circumference, the >35- and 18-35-year olds differed significantly. The older women
tend to have children with larger head circumferences. Moreover, in category non-smoking
only during pregnancy, the <18-year-olds had a significantly lower BMI than the
18-35-year-olds.
Finally, a linear model was performed to compare the newborn parameters from the
offspring of smoking and non-smoking mothers during pregnancy for each age category (Table 7). With increasing maternal age, the mean
differences of the parameters birth weight, birth length and head circumference increased,
between smoking and non-smoking mothers. Note that the means are adjusted by the maternal
parameters, body height, pre-pregnancy BMI and gestational weight gain.
Discussion
This study was designed to assess the effects of smoking during pregnancy on the foetal
development with special focus on the interaction with maternal age. This involved analysing
4142 singleton births from primiparous women from Vienna and compared newborn size as well
as vital parameters between mothers of different age groups and smoking behaviour.
Certain limitations of this study deserve mention. The socio-economic status of women was
not incorporated, although this status is often very meaningful in the context of birth and
pregnancy because it is an indicator of maternal and children's parameters (Lu et al. 2001; Phung
et al. 2003; Cnattingius 2004). Even with
an adequate health care system, socio-economic status also has a clear influence on birth
mode and birth complications (Kim et al. 2018).
Moreover, smoking behaviour was only surveyed and not controlled in any manner, raising some
uncertainty whether the information provided by the women is fully reliable since smoking is
socially undesirable during pregnancy, some underestimates about smoking behaviour and the
number of cigarettes smoked per day are possible. Finally, we have no information about
possible consumption of other substances, such as alcohol, that could influence on foetal
development (Jaddoe et al. 2007a). Note also the
relatively small representation of young (< 18 years old) and old mothers (> 35 years)
in the sample, especially when they are divided into the smoking subgroups. The data for
this study were collected from the early to mid-1990s and smoking behaviour in Austria has
changed in the meantime (Griebler et al. 2017).
Importantly, however, a change in smoking prevalence among women has no influence on the
effect of smoking during pregnancy on the foetal parameters. That effect remains negative.
The analysis of the newborn parameters showed that newborns of mothers who smoke before and
during pregnancy were significantly lighter, shorter and had a smaller head circumference
than newborns of non-smokers. This trend is consistent with previous studies (Kirchengast and Hartmann 2003; Kharkova and Odland 2019). This impaired foetal development by tobacco
consumption were explained by negative morphological and molecular changes in the placenta
(Zdravkovic et al. 2005). Breton et al. (2009) also showed
that epigenetic modifications that occur in children of mothers who smoked throughout
pregnancy can affect and impair foetal development. We could not find any significant
differences in newborn size between the children of non-smokers and those children whose
mothers stopped smoking during pregnancy. This indicates that smoking before pregnancy did
not have a significant effect on foetal development and growth. Thus, quitting smoking at
the beginning of pregnancy, seems to prevent foetal growth restriction. In the present
study, some women started smoking with pregnancy. The newborns of these mothers showed a
similar newborn size to those of mothers who smoked before and during pregnancy. Nafstad et al. (1996) reported a small but still considerable percentage (7%) of women who started
smoking with pregnancy, but those authors provided no information about the effects on
newborn size and vital parameters. To our knowledge, no previous study has investigated this
issue and this could be an interesting topic for future studies. APGAR 1 and APGAR 5 did not
differ significantly between smokers and non-smokers. We therefore omitted these two
parameters in our further analysis.
Multiple regression models showed that the number of smoked cigarettes per day had a
significant, independent effect on newborn parameters. A higher value had a significant
negative impact on birthweight, head circumference and birth length. Such a dose dependent
effect of nicotine consumption during pregnancy has also been shown by Jaddoe et al. (2008). The
model is controlled for the maternal parameters maternal age, body height, BMI and weight
gain during pregnancy because they also clearly influence child development.
Our study highlights that the negative effects of smoking during pregnancy on the newborn
size are amplified with increasing maternal age. Comparison of newborn parameters between
maternal age categories within each smoking group showed significant differences only in the
non-smoking group for birth weight and head circumference, i.e. these parameters increase
with maternal age. However, the trend that the offspring of older mothers weigh more and
have larger head circumferences seems to vanish if the mothers smoke during pregnancy. We
found no differences for any newborn parameter between mothers who smoked before and during
pregnancy and those who started smoking with pregnancy. Instead the values of the 18-35- and
over 35-year-olds are similarly low as the values of the adolescent mothers under 18 years
of age. This seems surprising because adolescent mothers tend to have smaller and lighter
children because their bodies are not fully developed yet and competition for nutrients
leads to smaller offspring compared to adult women (Kirchweger et al. 2018).
We then compared the newborn parameters of the smoking group and those of the non-smoking
group within each age category. As expected, the negative effects of nicotine consumption
increased during pregnancy. For birthweight the effect was most evident: the mean difference
increased from 101.8 g for the <18-year-olds to 245.8 g for the oldest mothers (>35
years of age). A similar pattern emerged for head circumference: a 0.3 cm mean difference
for the youngest and 18-35-year old mothers versus 0.7 cm for the older women. The effect is
somewhat weaker for birth length: a slight increase from 0.6 cm in the youngest group to 0.7
cm in the middle and oldest age group. These results are in line with the findings of Phung et al. (2003) and Zheng et al. (2016). The advantage of our study, however, is that we
controlled for confounding factors such as maternal stature, pre-pregnancy BMI and weight
gain during pregnancy, which have been shown to significantly influence birth weight (Pölzlberger et al. 2017). This makes our results more
accurate than those of previous studies.
Cnattingius (1997) reported a higher risk of small gestational age newborns (SGA) for older
mothers. He argued that this effect is not due to differences in smoking habits between
younger and older mothers, but rather to a different biological response to tobacco
consumption in older women. Possible explanations are on the one hand, that older women may
have smoked for a longer time than younger women and therefore the toxic tobacco substances
caused more damage to their organisms. On the other hand, increased maternal age is an
independent risk factor for adverse birth outcomes and restricted foetal growth (Cleary-Goldman et al. 2005; Salem Yaniv et al. 2011) as is smoking during pregnancy, as our study
has shown. Accordingly, the effects of advanced maternal age and nicotine consumption on the
developing foetus are apparently additive.
Other possibilities besides direct biological differences between younger and older
mothers, should also be addressed. The actual number of smoked cigarettes may differ between
age groups. Jaddoe et al. (2008) reported that the effect on the developing foetus gets worse with
a higher dose of smoked cigarettes per day. Even though the older mothers in our sample had
the highest percentages of non-smokers during pregnancy, this age category also had more
heavy smokers (>20 cigarettes per day). If older mothers are more likely to be heavy
smokers during pregnancy, this could lead to the observed increased negative effects on the
newborn parameters. Our study did not control for the number of cigarettes smoked per day
when comparing newborn parameters between age groups, because the sample sizes were too
small for the youngest and oldest mothers. Future studies should include the smoking dose in
their analysis.
Zheng et al. (2016) argued that the amplified effects of smoking on foetal development might not
be directly caused by age but rather by indirect factors linked to advanced maternal age.
Such possible age-related factors could be socio-economic status and educational level. Some
authors drew a correlation between smoking during pregnancy and the socio-economic status of
the women (Lu et al. 2001; Phung et al. 2003; Cnattingius
2004; Jaddoe et al. 2008; Tsakiridis et al. 2018; Wolff et al. 2019). More highly educated women are more likely to stop
smoking during pregnancy (Phung et al. 2003; Jaddoe et al. 2008) and the mean differences in birth
weight between smokers’ and non-smokers’ offspring decrease with educational level (Phung et al. 2003). Furthermore, low socio-economic
status is associated with a higher risk for adverse birth outcomes (Kim and Saada 2013). The question arises if socio-economic status of
smoking mothers differs between age groups. If yes, that could influence this interaction of
maternal age and the negative effects of nicotine consumption on newborn parameters.
Conclusion
The present study once again highlighted the negative effects of nicotine consumption
during pregnancy on the foetal development. Furthermore, our results indicate that the
effects of tobacco consumption during pregnancy are modified through the mother’s age. With
increasing age, the well-known negative effect of smoking during pregnancy increases, and
this is accompanied by stronger consequences for newborn size, especially for birth weight.
Our results show the importance of focusing on this high-risk group of older smoking mothers
during prenatal care. Especially against the background of increasing maternal age in our
society these findings are of special interest for public health systems and smoking
prevention programmes.
Appendix
Table 1 Sample characteristics
| Maternal parameters |
Mean (SD) |
Range |
N (%) |
| Age (years) |
25.2 (5.6) |
13-46 |
4142 |
|
<18 |
|
|
193 (4.7%) |
|
18-35 |
|
|
3734 (90.1%) |
|
>35 |
|
|
215 (5.2%) |
| Stature (cm)
|
163.7 (6.4) |
120-188 |
4105 |
| Pre-pregnancy weight (kg) |
60.5 (10.9) |
43-130 |
4142 |
| End of pregnancy weight (kg) |
73.4 (12.0) |
44-143 |
4142 |
| Gestational weight gain (kg) |
13.0 (5.5) |
-6-38 |
4142 |
| Pre-pregnancy body mass index
(kg/m2) |
22.55 (3.78) |
14.15-52.78 |
4105 |
| < 18.50 kg/m2 |
|
|
299 (7.3%) |
| 18.50 – 24.99 kg/m2 |
|
|
2989 (72.8%) |
| 25.00 – 29.99 kg/m2 |
|
|
635 (15.5%) |
| ≥ 30.00 kg/m2 |
|
|
179 (4.4%) |
| Nicotine
consumption before pregnancy |
|
|
|
| Smokers |
|
|
1495 (36.1%) |
| Non-smokers |
|
|
2647 (63.9%) |
| Nicotine
consumption during pregnancy |
|
|
|
| Smokers |
|
|
1186 (28.6%) |
| Non-smokers |
|
|
2156 (71.4%) |
|
|
|
|
| Newborn
parameters |
|
|
|
| Birth length (cm) |
49.9 (1.9) |
31-58 |
4137 |
| Birth weight (g) |
3386.3 (430.4) |
1800-5180 |
4142 |
| Head circumference (cm) |
34.4 (1.4) |
30-40 |
3815 |
| APGAR 1 |
8.6 (1.1) |
1-10 |
4107 |
| APGAR 5 |
9.8 (0.6) |
5-10 |
3878 |
Table 2 Maternal smoking behaviour during pregnancy per age category
| Age categories |
|
Smoking categories |
|
|
0 |
1 |
2 |
3 |
| <18 |
% |
47.7% |
13.5% |
31.1% |
7.8% |
|
N |
92 |
26 |
60 |
15 |
|
Expected N
|
113.9 |
23.9 |
45.8 |
9.5 |
|
Residuals |
-21.9 |
2.1 |
14.2 |
5.5 |
|
Standardised residuals |
-2.1 |
0.4 |
2.1 |
1.8 |
|
|
|
|
|
|
| 18-35 |
% |
58.9% |
12.6% |
23.7% |
4.8% |
|
N |
2200 |
470 |
886 |
178 |
|
Expected N
|
2203.3 |
461.6 |
886.2 |
183.0 |
|
Residuals |
-3.3 |
8.4 |
-0.2 |
-5.0 |
|
Standardised residuals |
-0.1 |
0.4 |
0.0 |
-0.4 |
|
|
|
|
|
|
| >35 |
% |
70.7% |
7.4% |
17.2% |
4.7% |
|
N |
152 |
16 |
37 |
10 |
|
Expected N
|
126.9 |
26.6 |
51.0 |
10.5 |
|
Residuals |
25.1 |
-10.6 |
-14.0 |
-0.5 |
|
Standardised residuals |
2.2 |
-2.1 |
-2.0 |
-0.2 |
Table 3 Comparison of maternal and newborn parameters between the four categories for
smoking behaviour during pregnancy Kruskal-Wallis H test
|
|
Maternal parameters |
Newborn parameters |
| Smoking categories |
|
Age a,b (years) |
Stature a,b,e (cm) |
BMI a,b
(kg/m²) |
Weight gain a,b,c (kg) |
Birth length b,c,d (cm) |
Birth weight b,d (g) |
Head circumference b (cm) |
APGAR
1 |
APGAR
5 |
| 0 |
N |
2444 |
2416 |
2416 |
2444 |
2441 |
2444 |
2259 |
2424 |
2283 |
| Mean |
25.6 |
163.3 |
22.66 |
12.3 |
50.1 |
3427.8 |
34.5 |
8.7 |
9.8 |
| SD |
5.6 |
6.4 |
3.64 |
5.3 |
1.8 |
420.0 |
1.4 |
1.1 |
0.6 |
| Median |
25 |
163 |
22.01 |
12 |
50 |
3400 |
34 |
9 |
10 |
| Q1/Q3 |
21/29 |
160/168 |
20.20/24.22 |
9/15.75 |
49/51 |
3150/3700 |
34/35 |
8/9 |
10/10 |
|
|
|
|
|
|
|
|
|
|
|
| 1 |
N |
512 |
508 |
508 |
512 |
510 |
512 |
476 |
508 |
459 |
| Mean |
24.4 |
164.9 |
22.35 |
14.0 |
50.0 |
3413.5 |
34.4 |
8.6 |
9.7 |
| SD |
5.1 |
6.0 |
3.74 |
5.5 |
1.8 |
430.9 |
1.3 |
1.3 |
0.6 |
| Median |
24 |
165 |
21.45 |
14 |
50 |
3400 |
34 |
9 |
10 |
| Q1/Q3 |
21/27 |
161/169 |
19.99/23.79 |
11/17 |
49/51 |
3150/3700 |
33/35 |
8/9 |
10/10 |
|
|
|
|
|
|
|
|
|
|
|
| 2 |
N |
983 |
979 |
979 |
983 |
983 |
983 |
898 |
975 |
936 |
| Mean |
24.4 |
164.1 |
22.42 |
13.7 |
49.4 |
3279.6 |
34.2 |
8.6 |
9.8 |
| SD |
5.5 |
6.2 |
4.12 |
5.4 |
2.0 |
440.3 |
1.4 |
1.2 |
0.6 |
| Median |
23 |
164 |
21.30 |
14 |
50 |
3300 |
34 |
9 |
10 |
| Q1/ Q3 |
20/28 |
160/168 |
19.61/24.22 |
10/17 |
48/51 |
3000/3550 |
33/35 |
8/9 |
10/10 |
|
|
|
|
|
|
|
|
|
|
|
| 3 |
N |
203 |
202 |
202 |
203 |
203 |
203 |
182 |
200 |
200 |
| Mean |
24.7 |
163.3 |
22.43 |
14.4 |
49.6 |
3334.4 |
34.2 |
8.6 |
9.8 |
| SD |
5.8 |
6.7 |
3.81 |
6.7 |
1.8 |
411.9 |
1.3 |
1.1 |
0.5 |
| Median |
24 |
163 |
21.62 |
14 |
50 |
3350 |
34 |
9 |
10 |
| Q1/ Q3 |
20/29 |
159/168 |
19.92/23.94 |
10/19 |
48/51 |
3070/3600 |
33/35 |
8/9 |
10/10 |
|
H |
49.347*** |
33.454*** |
18.852*** |
82.883*** |
91.525*** |
82.176*** |
42.097*** |
2.570 |
2.010 |
Table 4 Associations between the newborn parameters birth weight, birth length and head
circumference, and the maternal parameters age, nicotine consumption during pregnancy.
Multiple regression analyses.
|
Birth weight (R²=0.148) |
| Independent variables |
B |
SE of B |
β |
p |
| Maternal age |
5.253 |
1.134 |
0.068 |
≤ 0.001 |
| Maternal stature |
12.082 |
0.985 |
0.179 |
≤ 0.001 |
| Weight gain during pregnancy |
17.053 |
1.158 |
0.217 |
≤ 0.001 |
| Maternal BMI |
24.564 |
1.681 |
0.216 |
≤ 0.001 |
| Nicotine consumption during pregnancy |
-11.640 |
1.039 |
-0.162 |
≤ 0.001 |
|
|
Birth length (R²=0.114) |
|
B |
SE of B |
β |
p |
| Maternal age |
0.020 |
0.005 |
0.059 |
≤ 0.001 |
| Maternal stature |
0.055 |
0.004 |
0.187 |
≤ 0.001 |
| Weight gain during pregnancy |
0.056 |
0.005 |
0.163 |
≤ 0.001 |
| Maternal BMI |
0.078 |
0.007 |
0.157 |
≤ 0.001 |
| Nicotine consumption during pregnancy |
-0.054 |
0.005 |
-0.171 |
≤ 0.001 |
|
|
Head circumference (R²=0.079) |
|
B |
SE of B |
β |
p |
| Maternal age |
0.019 |
0.004 |
0.075 |
≤ 0.001 |
| Maternal stature |
0.033 |
0.003 |
0.150 |
≤ 0.001 |
| Weight gain during pregnancy |
0.029 |
0.004 |
0.114 |
≤ 0.001 |
| Maternal BMI |
0.059 |
0.006 |
0.161 |
≤ 0.001 |
| Nicotine consumption during pregnancy |
-0.029 |
0.004 |
-0.124 |
≤ 0.001 |
Table 5 Maternal somatic parameters per smoking category and per age category.
Kruskal-Wallis H-test
|
|
Smoking categories |
|
|
0 |
1 |
2 |
3 |
|
|
Age categories (years) |
Age categories (years) |
Age categories (years) |
Age categories (years) |
|
|
<18 |
18-35 |
>35 |
<18 |
18-35 |
>35 |
<18 |
18-35 |
>35 |
<18 |
18-35 |
>35 |
| Stature (cm) |
N |
92 |
2172 |
152 |
26 |
467 |
15 |
60 |
882 |
37 |
15 |
177 |
10 |
|
Mean |
161.3 |
163.4 |
162.5 |
165.7 |
164.8 |
165.9 |
162.9 |
164.1 |
166.0 |
162.6 |
163.4 |
164 |
|
SD |
5.9 |
6.4 |
6.7 |
5.5 |
6.1 |
4.2 |
6.1 |
6.2 |
7.2 |
6.0 |
6.8 |
6.9 |
|
Median |
160.5 |
164 |
163 |
166 |
165 |
166 |
162.5 |
164 |
165 |
162 |
163 |
163 |
|
Q1/Q3 |
157.3/165 |
160/168 |
158/167 |
162/170 |
161/169 |
164/169 |
158.5/166.5 |
160/168 |
161/172 |
160/165 |
159/168 |
160/169.3 |
| Kruskal-Wallis H test |
H |
13.353*a |
1.337 |
5.914 |
0.252 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BMI (kg/m²) |
N |
92 |
2172 |
152 |
26 |
467 |
15 |
60 |
882 |
37 |
15 |
177 |
10 |
|
Mean |
21.61 |
22.60 |
24.22 |
20.88 |
22.40 |
23.13 |
21.47 |
22.44 |
23.55 |
21.75 |
22.45 |
23.24 |
|
SD |
2.47 |
3.61 |
4.37 |
3.37 |
3.71 |
4.64 |
3.22 |
4.13 |
4.92 |
1.51 |
4.0 |
2.20 |
|
Median |
21.41 |
21.94 |
23.10 |
20.67 |
21.48 |
21.09 |
20.64 |
21.31 |
22.19 |
21.48 |
21.38 |
23.67 |
|
Q1/Q3 |
19.71/
22.95 |
20.20/
24.21 |
21.23/
26.03 |
18.40/
21.89 |
20.05/
23.88 |
20.55/
24.80 |
19.33/
22.79 |
19.61/
24.22 |
20.54/
25.28 |
20.57/
22.58 |
19.72/
24.01 |
22.03/
24.86 |
| Kruskal-Wallis H test |
H |
31.658*a,b |
6.160*a |
5.958 |
2.496 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Weight gain (kg) |
N |
92 |
2200 |
152 |
26 |
470 |
16 |
60 |
886 |
37 |
15 |
178 |
10 |
|
Mean |
12.3 |
12.4 |
11.5 |
11.8 |
14.1 |
14.1 |
13.4 |
13.7 |
13.2 |
16.2 |
14.1 |
15.6 |
|
SD |
5.3 |
5.3 |
5.6 |
6.0 |
5.5 |
6.1 |
5.4 |
5.4 |
5.1 |
8.4 |
6.5 |
8.2 |
|
Median |
12.0 |
12 |
12 |
12 |
14 |
13.5 |
13 |
14 |
14 |
15 |
14 |
12.5 |
|
Q1/Q3 |
9.0/16.0 |
9/16 |
8/15 |
8.8/15.3 |
11/17.3 |
10.5/18.8 |
10/16.8 |
10/17 |
9.5/17.5 |
9/22 |
10/18.3 |
11.8/21.8 |
| Kruskal-Wallis H test |
H |
4.210 |
3.153 |
0.654 |
0.241 |
Table 6 Newborn parameters per smoking category and per age category. Kruskal-Wallis
H-test
|
Smoking category |
|
|
0 |
1 |
2 |
3 |
|
|
Age categories (years) |
Age categories (years) |
Age categories (years) |
Age categories (years) |
|
|
<18 |
18-35 |
>35 |
<18 |
18-35 |
>35 |
<18 |
18-35 |
>35 |
<18 |
18-35 |
>35 |
| Birth length (cm) |
N |
92 |
2198 |
151 |
26 |
468 |
16 |
60 |
886 |
37 |
15 |
178 |
10 |
| Mean |
50 |
50.1 |
50.3 |
49.6 |
50 |
50.1 |
49.3 |
49.4 |
50 |
50.1 |
49.5 |
49.3 |
| SD |
1.5 |
1.8 |
1.8 |
1.5 |
1.8 |
1.7 |
1.8 |
2 |
2.1 |
1.9 |
1.8 |
2.7 |
| Median |
50 |
50 |
50 |
49.5 |
50 |
50 |
49 |
50 |
50 |
50 |
50 |
50 |
| Q1/Q3 |
49/51 |
49/51 |
49/51 |
49/51 |
49/51 |
49.3/51 |
48/51 |
48/51 |
48/51 |
49/51 |
48/51 |
48.5/50.3 |
| Kruskal-Wallis H test |
H |
3.159 |
1.960 |
2.389 |
1.048 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Birth weight (g) |
N |
92 |
2200 |
152 |
26 |
470 |
16 |
60 |
886 |
37 |
15 |
178 |
10 |
| Mean |
3310.2 |
3428.2 |
3492.5 |
3302.7 |
3417.9 |
3465.6 |
3216.3 |
3282.7 |
3307.6 |
3362.7 |
3340.5 |
3184 |
| SD |
370.4 |
420.9 |
423.4 |
363 |
430.9 |
525.9 |
418.1 |
439.9 |
488.5 |
496.7 |
398.8 |
520.2 |
| Median |
3275 |
3400 |
3500 |
3300 |
3400 |
3500 |
3300 |
3300 |
3300 |
3350 |
3350 |
3350 |
| Q1/Q3 |
3012.5/
3547.5 |
3150/
3700 |
3200/
3800 |
3150/
3525 |
3137.5/
3700 |
3162.5/
3762.5 |
2900/
3550 |
3000/
3550 |
2990/
3570 |
3100/
3600 |
3050/
3650 |
3025/
3500 |
| Kruskal-Wallis H test |
H |
13.160*a,b |
2.049 |
0.710 |
0.380 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Head
circumference
(cm) |
N |
80 |
2043 |
136 |
23 |
440 |
13 |
59 |
802 |
37 |
15 |
158 |
9 |
| Mean |
34 |
34.5 |
34.8 |
34.1 |
34.4 |
34.5 |
33.9 |
34.2 |
34.3 |
33.9 |
34.3 |
34.4 |
| SD |
1.2 |
1.4 |
1.3 |
1.2 |
1.3 |
1.3 |
1.5 |
1.4 |
1.4 |
1.4 |
1.3 |
1.2 |
| Median |
34 |
34 |
35 |
34 |
34 |
35 |
34 |
34 |
34 |
34 |
34 |
35 |
| Q1/Q3 |
33/35 |
34/35 |
34/35 |
33/35 |
33/35 |
34/35 |
33/35 |
33/35 |
33/35 |
33/35 |
33/35 |
33/35 |
| Kruskal-Wallis H test |
H |
18.689*a,b,c |
1.241 |
3.097 |
1.381 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Apgar 1 |
N |
92 |
2180 |
152 |
26 |
466 |
16 |
60 |
879 |
36 |
14 |
176 |
10 |
| Mean |
8.5 |
8.7 |
8.6 |
8.65 |
8.6 |
8.7 |
8.7 |
8.6 |
8.8 |
8.6 |
8.6 |
8.8 |
| SD |
1.1 |
1.1 |
1.3 |
1.2 |
1.3 |
1.3 |
0.8 |
1.2 |
1.2 |
0.7 |
1.2 |
0.9 |
| Median |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
8.5 |
9 |
9 |
| Q1/Q3 |
8/9 |
8/9 |
8/9 |
8/9 |
8/9 |
8.3/9.8 |
8/9 |
8/9 |
9/9 |
8/9 |
8/9 |
8/9.3 |
| Kruskal-Wallis H test |
H |
3.960 |
0.875 |
1.549 |
1.015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Apgar 5 |
N |
88 |
2055 |
140 |
24 |
419 |
16 |
58 |
842 |
36 |
14 |
176 |
10 |
| Mean |
9.7 |
9.8 |
9.7 |
9.67 |
9.7 |
9.9 |
9.8 |
9.8 |
9.9 |
9.8 |
9.7 |
9.9 |
| SD |
0.6 |
0.6 |
0.7 |
0.7 |
0.7 |
0.3 |
0.5 |
0.6 |
0.3 |
0.4 |
0.6 |
0.3 |
| Median |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
| Q1/Q3 |
9.3/10 |
10/10 |
10/10 |
9.3/10 |
10/10 |
10/10 |
10/10 |
10/10 |
10/10 |
9.8/10 |
10/10 |
10/10 |
| Kruskal-Wallis H test |
H |
3.145 |
0.985 |
1.894 |
0.752 |
Table 7 Mean values for birth weight, birth length and head circumference for smokers
and non-smokers during pregnancy and their adjusted mean differences (95% CI)
|
Birth weight (g) |
|
|
Non-smokers |
smokers |
|
|
| Age categories |
Mean (SD) |
N |
Mean (SD) |
N |
Adjusted mean difference (95% CI)a |
p-value |
| <18 years |
3308.6 (367.2) |
118 |
3245.6 (435.4) |
75 |
101.8 (-9.0 - 212.6) |
0.072 b,c,d |
| 18-35 years |
3426.4 (422.6) |
2670 |
3292.4 (433.6) |
1064 |
154.9 (126.4 - 183.5) |
<0,000 b,c,d |
| >35 years |
3490.0 (432.4) |
168 |
3281.3 (492.3) |
47 |
254.8 (115.8 - 393.8) |
<0,000 b,c,d |
|
|
|
|
|
|
|
|
Birth length (cm) |
|
|
Non-smokers |
Smokers |
|
|
|
Mean (SD) |
N |
Mean (SD) |
N |
Adjusted mean difference (95% CI)a |
p-value |
| <18 years |
49.9 (1.5) |
118 |
49.5 (1.8) |
75 |
0.6 (0.1 – 1.0) |
0.013 b,c,d |
| 18-35 years |
50.1 (1.8) |
2666 |
49.4 (2.0) |
1064 |
0.7 (0.6 - 0.9) |
<0.000 b,c,d |
| >35 years |
50.3 (1.8) |
167 |
49.8 (2.2) |
47 |
0.7 (0.1-1.3) |
0.034 d |
|
|
|
|
|
|
|
|
Head circumference (cm) |
|
|
Non-smokers |
Smokers |
|
|
|
Mean (SD) |
N |
Mean (SD) |
N |
Adjusted mean difference (95% CI)a |
p-value |
| <18 years |
34.1 (1.2) |
103 |
33.9 (1.4) |
74 |
0.3 (-0.1 - 0.7) |
0.161 b,c,d |
| 18-35 years |
34.5 (1.4) |
2483 |
34.2 (1.4) |
960 |
0.3 (0.2 - 0.4) |
<0.000 b,c,d |
| >35 years |
34.8 (1.3) |
149 |
34.3 (1.3) |
46 |
0.6 (0.2 - 1.0) |
0.008 c,d |
References
Aliyu, Muktar H./Salihu, Hamisu M./Wilson, Ronee
E./Alio, Amina P./Kirby, Russell S. (2008). The risk of intrapartum stillbirth among
smokers of advanced maternal age. Archives of Gynecology and Obstetrics 278 (1), 39–45.
https://doi.org/10.1007/s00404-007-0529-8.
Breton, C. V./Byun, H.-M./Wenten, M./Pan, F./Yang,
A./Gilliland, F. D. (2009). Prenatal tobacco smoke exposure affects global and
gene-specific DNA methylation. American journal of respiratory and critical care
medicine 180 (5), 462–467. https://doi.org/10.1164/rccm.200901-0135OC.
Briggs, M. M./Hopman, W. M./Jamieson, M. A.
(2007). Comparing Pregnancy in Adolescents and Adults: Obstetric Outcomes and Prevalence
of Anemia. Journal of Obstetrics and Gynaecology Canada 29 (7), 546–555. https://doi.org/10.1016/S1701-2163(16)32506-3.
Casey, B. M./McIntire, D. D./Leveno, K. J. (2001).
The continuing value of the Apgar score for the assessment of newborn infants. The New
England Journal of Medicine 344 (7), 467–471. https://doi.org/10.1056/nejm200102153440701.
Cleary-Goldman, J./Malone, F. D./Vidaver, J./Ball,
R. H./Nyberg, D. A./Comstock, C. H./Saade, G. R./Eddleman, K. A./Klugman, S./Dugoff,
L./Timor-Tritsch, I. E./Craigo, S. D./Carr, S. R./Wolfe, H. M./Bianchi, D. W./D'Alton,
M. (2005). Impact of maternal age on obstetric outcome. Obstetrics and Gynecology 105 (5
Pt 1), 983–990. https://doi.org/10.1097/01.AOG.0000158118.75532.51.
Cnattingius, S. (1990). Does age potentiate the
smoking-related risk of fetal growth retardation? Obstetrical & Gynecological Survey
45 (9), 606. https://doi.org/10.1097/00006254-199009000-00009.
Cnattingius, S. (1997). Maternal age modifies the
effect of maternal smoking on intrauterine growth retardation but not on late fetal
death and placental abruption. American Journal of Epidemiology 145 (4), 319–323.
https://doi.org/10.1093/oxfordjournals.aje.a009108.
Cnattingius, S. (2004). The epidemiology of
smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy
outcomes. Nicotine & Tobacco Research : Official Journal of the Society for Research
on Nicotine and Tobacco 6 Suppl 2, S125-40. https://doi.org/10.1080/14622200410001669187.
Cnattingius, S. M. D./Axelsson, O. M. D./Eklund,
G./Lindmark, G. M. D. (1985). Smoking, maternal age, and fetal growth. Obstetrics &
Gynecology 66 (4), 449–452.
Dulitzki, M. (1998). Effect of very advanced
maternal age on pregnancy outcome and rate of cesarean delivery. Obstetrics and
Gynecology 92 (6), 935–939. https://doi.org/10.1016/s0029-7844(98)00335-4.
Ekblad, M./Korkeila, J./Lehtonen, L. (2015).
Smoking during pregnancy affects foetal brain development. Acta Paediatrica 104 (1),
12–18. https://doi.org/10.1111/apa.12791.
Fitzpatrick, K. E./Tuffnell, D./Kurinczuk, J.
J./Knight, M. (2017). Pregnancy at very advanced maternal age: a UK population-based
cohort study. BJOG : An International Journal of Obstetrics and Gynaecology 124 (7),
1097–1106. https://doi.org/10.1111/1471-0528.14269.
Griebler, R./Winkler, P./Gaiswinkler, S./Delcour,
J./Juraszovich, B./Nowotny, M./Pochobradsky, E./Schleicher, B./Schmutterer, I. (2017).
Österreichischer Gesundheitsbericht 2016. Berichtszeitraum 2005–2014/2015.
Bundesministerium für Gesundheit und Frauen. Wien.
Gueri, M./Jutsum, P./Sorhaindo, B. (1982).
Anthropometric assessment of nutritional status in pregnant women: a reference table of
weight-for-height by week of pregnancy. The American Journal of Clinical Nutrition 35
(3), 609–616. https://doi.org/10.1093/ajcn/35.3.609.
Hoffman, M. C./Jeffers, S./Carter, J./Duthely,
L./Cotter, A./González-Quintero, V. H. (2007). Pregnancy at or beyond age 40 years is
associated with an increased risk of fetal death and other adverse outcomes. American
Journal of Obstetrics and Gynecology 196 (5), e11-3. https://doi.org/10.1016/j.ajog.2006.10.862.
Huang, L./Sauve, R./Birkett, N./Fergusson, D./van
Walraven, C. (2008). Maternal age and risk of stillbirth: a systematic review. CMAJ :
Canadian Medical Association Journal = journal de l'Association medicale canadienne 178
(2), 165–172. https://doi.org/10.1503/cmaj.070150.
Jacobsson, B./Ladfors, L./Milsom, I. (2004).
Advanced maternal age and adverse perinatal outcome. Obstetrics and Gynecology 104 (4),
727–733. https://doi.org/10.1097/01.aog.0000140682.63746.be.
Jaddoe, V. W. V./Bakker, R./Hofman, A./Mackenbach,
J. P./Moll, H. A./Steegers, E. A. P./Witteman, J. C. M. (2007a). Moderate alcohol
consumption during pregnancy and the risk of low birth weight and preterm birth: the
generation R study. Annals of Epidemiology 17 (10), 834–840. https://doi.org/10.1016/j.annepidem.2007.04.001.
Jaddoe, V. W. V./Troe, E.-J. W. M./Hofman,
A./Mackenbach, J. P./Moll, H. A./Steegers, E. A. P./Witteman, J. C. M. (2008). Active
and passive maternal smoking during pregnancy and the risks of low birthweight and
preterm birth: the Generation R Study. Paediatric and Perinatal Epidemiology 22 (2),
162–171. https://doi.org/10.1111/j.1365-3016.2007.00916.x.
Jaddoe, V. W. V./Verburg, B. O./Ridder, M. A. J.
de/Hofman, A./Mackenbach, J. P./Moll, H. A./Steegers, E. A. P./Witteman, J. C. M.
(2007b). Maternal smoking and fetal growth characteristics in different periods of
pregnancy: the generation R study. American Journal of Epidemiology 165 (10), 1207–1215.
https://doi.org/10.1093/aje/kwm014.
Jolly, M./Sebire, N./Harris, J./Robinson,
S./Regan, L. (2000). The risks associated with pregnancy in women aged 35 years or
older. Human Reproduction 15 (11), 2433–2437. https://doi.org/10.1093/humrep/15.11.2433.
Kahveci, B./Melekoglu, R./Evruke, I. C./Cetin, C.
(2018). The effect of advanced maternal age on perinatal outcomes in nulliparous
singleton pregnancies. BMC Pregnancy and Childbirth 18 (1), 343. https://doi.org/10.1186/s12884-018-1984-x.
Kenny, L. C./Lavender, T./McNamee, R./O'Neill, S.
M./Mills, T./Khashan, A. S. (2013). Advanced maternal age and adverse pregnancy outcome:
evidence from a large contemporary cohort. PloS One 8 (2), e56583. https://doi.org/10.1371/journal.pone.0056583.
Kharkova, O./Odland, J. (2019). Effect of smoking
behaviour before and during pregnancy on low head circumference at birth. European
Journal of Public Health 29 (Supplement_4). https://doi.org/10.1093/eurpub/ckz187.126.
Kim, D./Saada, A. (2013). The social determinants
of infant mortality and birth outcomes in Western developed nations: a cross-country
systematic review. International Journal of Environmental Research and Public Health 10
(6), 2296–2335. https://doi.org/10.3390/ijerph10062296.
Kim, M. K./Lee, S. M./Bae, S.-H./Kim, H. J./Lim,
N. G./Yoon, S.-J./Lee, J. Y./Jo, M.-W. (2018). Socioeconomic status can affect pregnancy
outcomes and complications, even with a universal healthcare system. International
Journal for Equity in Health 17 (1), 2. https://doi.org/10.1186/s12939-017-0715-7.
Kirchengast, S./Hartmann, B. (2003). Nicotine
consumption before and during pregnancy affects not only newborn size but also birth
modus. Journal of Biosocial Science 35 (2), 175–188. https://doi.org/10.1017/S0021932003001755.
Kirchweger, F./Kirchengast, S./Hafner,
E./Stümpflein, I./Hartmann, B. (2018). The impact of maternal age on foetal growth
patterns and newborn size. Anthropological Review 81 (2), 111–129. https://doi.org/10.2478/anre-2018-0009.
Knopik, V. S./Maccani, M. A./Francazio,
S./McGeary, J. E. (2012). The epigenetics of maternal cigarette smoking during pregnancy
and effects on child development. Development and Psychopathology 24 (4), 1377–1390.
https://doi.org/10.1017/S0954579412000776.
Knußmann, R. (1988). Somatometrie. In: R. Knußmann
(Ed.). Anthropologie. Handbuch der vergleichenden Biologie des Menschen ; zugleich 4.
Auflage des Lehrbuchs der Anthropologie, begründet von Rudolf Martin. Stuttgart/Jena/New
York, G. Fischer.
Lamminpää, R./Vehviläinen-Julkunen, K./Gissler,
M./Heinonen, S. (2013). Smoking among older childbearing women - a marker of risky
health behaviour a registry-based study in Finland. BMC Public Health 13, 1179.
https://doi.org/10.1186/1471-2458-13-1179.
Lu, Y./Tong, S./Oldenburg, B. (2001). Determinants
of smoking and cessation during and after pregnancy. Health Promotion International 16
(4), 355–365. https://doi.org/10.1093/heapro/16.4.355.
Mook-Kanamori, D. O./Steegers, E. A. P./Eilers, P.
H./Raat, H./Hofman, A./Jaddoe, V. W. V. (2010). Risk factors and outcomes associated
with first-trimester fetal growth restriction. JAMA 303 (6), 527–534. https://doi.org/10.1001/jama.2010.78.
Nafstad, P./Botten, G./Hagen, J. (1996). Partner's
smoking: a major determinant for changes in women's smoking behaviour during and after
pregnancy. Public Health 110 (6), 379–385. https://doi.org/10.1016/S0033-3506(96)80012-6.
Ogawa, K./Urayama, K. Y./Tanigaki, S./Sago,
H./Sato, S./Saito, S./Morisaki, N. (2017). Association between very advanced maternal
age and adverse pregnancy outcomes: a cross sectional Japanese study. BMC Pregnancy and
Childbirth 17 (1), 349. https://doi.org/10.1186/s12884-017-1540-0.
Phung, H./Bauman, A./Nguyen, T. V./Young, L./Tran,
M./Hillman, K. (2003). Risk factors for low birth weight in a socio-economically
disadvantaged population: parity, marital status, ethnicity and cigarette smoking.
European Journal of Epidemiology 18 (3), 235–243. https://doi.org/10.1023/A:1023384213536.
Pölzlberger, E./Hartmann, B./Hafner,
E./Stümpflein, I./Kirchengast, S. (2017). Maternal height and pre-pregnancy weight
status are associated with fetal growth patterns and newborn size. Journal of Biosocial
Science 49 (3), 392–407. https://doi.org/10.1017/S0021932016000493.
Prabhu, N./Smith, N./Campbell, D./Craig, L.
C./Seaton, A./Helms, P. J./Devereux, G./Turner, S. W. (2010). First trimester maternal
tobacco smoking habits and fetal growth. Thorax 65 (3), 235–240. https://doi.org/10.1136/thx.2009.123232.
Radoń-Pokracka, M./Adrianowicz, B./Płonka,
M./Danił, P./Nowak, M./Huras, H. (2019). Evaluation of pregnancy outcomes at advanced
maternal age. Open Access Macedonian Journal of Medical Sciences 7 (12), 1951–1956.
https://doi.org/10.3889/oamjms.2019.587.
Salem Yaniv, S./Levy, A./Wiznitzer, A./Holcberg,
G./Mazor, M./Sheiner, E. (2011). A significant linear association exists between
advanced maternal age and adverse perinatal outcome. Archives of Gynecology and
Obstetrics 283 (4), 755–759. https://doi.org/10.1007/s00404-010-1459-4.
Shan, D./Qiu, P.-Y./Wu, Y.-X./Chen, Q./Li,
A.-L./Ramadoss, S./Wang, R.-R./Hu, Y.-Y. (2018). Pregnancy outcomes in women of advanced
maternal age: a retrospective cohort study from China. Scientific Reports 8 (1), 12239.
https://doi.org/10.1038/s41598-018-29889-3.
Statistik Austria (Hrsg.) (2019). Statistisches
Jahrbuch 2019.
Toledo-Rodriguez, M/Lotfipour, S./Leonard,
G./Perron, M./Richer, L./Veillette, S./Pausova, Z./Paus, T. (2010). Maternal smoking
during pregnancy is associated with epigenetic modifications of the brain-derived
neurotrophic factor-6 exon in adolescent offspring. American Journal of Medical
Genetics. Part B, Neuropsychiatric Genetics : The Official Publication of the
International Society of Psychiatric Genetics 153B (7), 1350–1354. https://doi.org/10.1002/ajmg.b.31109.
Tromp, M./Ravelli, A. C. J./Reitsma, J. B./Bonsel,
G. J./Mol, B. W. (2011). Increasing maternal age at first pregnancy planning: health
outcomes and associated costs. Journal of Epidemiology and Community Health 65 (12),
1083–1090. https://doi.org/10.1136/jech.2009.095422.
Tsakiridis, I./Mamopoulos, A./Papazisis,
G./Petousis, S./Liozidou, A./Athanasiadis, A./Dagklis, T. (2018). Prevalence of smoking
during pregnancy and associated risk factors: a cross-sectional study in Northern
Greece. European Journal of Public Health 28 (2), 321–325. https://doi.org/10.1093/eurpub/cky004.
Voigt, M./Briese, V./Jorch, G./Henrich,
W./Schneider, K. T. M./Straube, S. (2009). The influence of smoking during pregnancy on
fetal growth: considering daily cigarette consumption and the SGA rate according to
length of gestation. Zeitschrift fur Geburtshilfe und Neonatologie 213 (5), 194–200.
https://doi.org/10.1055/s-0029-1214405.
Wilkie, J. R. (1981). The trend toward delayed
parenthood. Journal of Marriage and the Family 43 (3), 583. https://doi.org/10.2307/351759.
Wolff, M. G. de/Backhausen, M. G./Iversen, M.
L./Bendix, J. M./Rom, A. L./Hegaard, H. K. (2019). Prevalence and predictors of maternal
smoking prior to and during pregnancy in a regional Danish population: a cross-sectional
study. Reproductive Health 16 (1), 82. https://doi.org/10.1186/s12978-019-0740-7.
Zdravkovic, T./Genbacev, O./McMaster, M.
T./Fisher, S. J. (2005). The adverse effects of maternal smoking on the human placenta:
a review. Placenta 26 Suppl A, S81-6. https://doi.org/10.1016/j.placenta.2005.02.003.
Zheng, W./Suzuki, K./Tanaka, T./Kohama,
M./Yamagata, Z. (2016). Association between maternal smoking during pregnancy and low
birthweight: effects by maternal age. PloS One 11 (1), e0146241. https://doi.org/10.1371/journal.pone.0146241.
