The impact of lifestyle factors and chronic stress on the frequency and
intensity of migraine and tension-type headaches among Austrian women
Sylvia Kirchengast ✉
✉
Department of Evolutionary Anthropology, University of Vienna, Austria
Department of Evolutionary Anthropology, University of Vienna, Austria
DOI: https://doi.org/10.52905/hbph2022.1.33
Abstract
BackgroundPrimary headache disorders such as migraine and tension-type headaches represent an
important public health problem. Besides genetic factors, environmental parameters, and,
in particular, recent lifestyle patterns may contribute to the increasing prevalence of
headache disorders.
Sample and methods173 women, between the ages of 18 and 65 years, were enrolled in the present study. The
present study focuses on the association between recent lifestyle patterns such as
physical activity, time spent outdoors, time spent in front of TV or computer screens,
nicotine consumption, weight status as well as chronic stress exposure, and the duration
and intensity of migraine and tension-type headaches (TTHs) in a female sample from
Austria. An extensive online questionnaire, consisting of 72 questions regarding
sociodemographic background, headache anamnesis, lifestyle factors, such as sleep, and
physical activity patterns, was distributed via online platforms by means of a snowball
sampling system. Additionally, chronic stress was measured using the Trier Inventory for
Chronic Stress.
ResultsParticipants suffering from migraine were significantly older than women suffering from
tension-type headaches (TTHs) and migraine. Age was significantly associated with
migraine attack frequency. Women suffering from tension-type headaches (TTH) intensity
showed significant associations with physical exercise, sleep, and chronic stress. Fewer
hours of exercise and higher stress were connected with higher pain intensity. Migraine
frequency, in contrast, correlated with daily hours spent in front of computer screen,
while stress and physical exercise did not show associations with migraines.
ConclusionThe study showed that lifestyle factors may be associated with and may have effects on
primary headache disorders, especially tension-type headaches.
Keywords: migraine, tension-type headache, lifestyle factors, chronic stress
Conflict of Interest: There are no
conflicts of interest.
Citation: Molnar, A. / Kirchengast, S. (2022). The impact of lifestyle factors and chronic stress on the frequency and
intensity of migraine and tension-type headaches among Austrian women. Human Biology and Public Health 1. https://doi.org/10.52905/hbph2022.1.33.
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 08-03-2022 | Accepted: 23-05-2022 | Published: 14-09-2022
Take home message for students
Primary headache disorders are not only painful for affected individuals, they also
represent a serious public health problem. Recent lifestyle patterns, such as chronic
stress, a lack of sleep, and a lack of physical activity seem to enhance the risk of
suffering from primary headaches.
Contents
Introduction
Currently, more than 45% of the global adult population suffers from active
headaches, the most common disorders of the neuro-system (Stovner et al. 2007). We have to distinguish between primary headaches,
which have no known underlying cause, and secondary headaches, which are symptoms of other
diseases (Headache Classification Committee of the
International Headache Society 2018). The present study focuses on primary headache
disorders which include several forms of migraines, tension-type headaches (TTHs), and
trigeminal autonomic cephalalgias such as cluster headaches (Headache Classification Committee of the International Headache Society
2018). According to the Global Burden of Diseases, Injuries, and Risk Factors (GBD)
studies (Vos et al. 2020), in the year 2016 nearly
three billion individuals worldwide were estimated to suffer from the most common primary
headache disorders, i.e. tension-type headaches (1.9 billion cases) or migraine (1.4 billion
cases). Although primary headaches are among the most prevalent disorders worldwide (Stovner et al. 2007), they are not fatal and nearly
everybody experiences headaches from time to time (Vos
et al. 2020). Perhaps these are reasons why the importance of headaches as a major
public health problem has only been recognized in the last 20 years (Vos et al. 2020). Nevertheless, GBD ranks headache disorders under the
top ten of the most disabling diseases. This is due to the fact, that the most common
primary headaches, i.e. migraine and chronic TTH, cause not only somatic but also
psychological pain and, consequently, a marked decrease in the quality of life (Lipton et al. 2000). Furthermore, several studies have
shown that migraines and TTHs have a profound negative impact on the larger economic
situation (Hamelsky et al. 2005; Linde and Dahlöf 2004; Lipton et al. 2003; Lipton et al.
2000). These headache disorders cause direct costs for the public health care
systems and also indirect costs due to work absences and reduced work efficiency (Jensen and Stovner 2008). These costs are expected to
continue to rise in the future, since a significant increase in the prevalence of headaches
has been observed over the past three decades. Lyngberg et al. (Lyngberg et al. 2005) reported a significant increase in TTH prevalence
from 1989 to 2001 among the Danish population. Additionally, the prevalence of migraine
increased in that 12-year period. This trend continues in other nations (Vos et al. 2020). The prevalence of primary headaches
varies between different geographic regions. The Eurolight project, studying 8.000 residents
from 10 European countries detected a headache lifetime prevalence of 91% and a 1-year
prevalence of 79% (Steiner et al. 2014). In
general, the prevalence of primary headaches in Europe is about 50%, which is the same as in
Asia, Australia, and North America, while only 20% of the African population is affected
(Stovner et al. 2007).
In order to develop strategies to reduce the prevalence of headaches, it is necessary to
understand its aetiology and identify risk factors. There is no doubt that primary headaches
have a strong genetic component (Sutherland et al.
2019), however, environmental factors influence the intensity and frequency of
headache diseases, too. Often cited trigger factors are weather changes or alterations of
barometric pressure, although various studies have yielded controversial results (Marrelli et al. 1988; Mukamal et al. 2009). Distinct intrinsic weather components, such as temperature,
sunshine duration, wind speed, humidity, and seasonal factors seem to enhance headache
(Yang et al. 2011). Furthermore, muscle tension,
neck pain (Tolentino et al. 2018), and menstrual
factors (Arjona et al. 2007; Campelo et al. 2021; van Casteren
et al. 2021) have been reported to trigger primary headaches. The focus of the
present study, however, lies on lifestyle factors and their impact on headache
characteristics.
The interaction of lifestyle and headaches is not only interesting from a public health
point of view, but also from an anthropological one. From an evolutionary perspective, we
have to be aware that the recent lifestyle differs drastically from that of our ancestors,
who lived in the so-called environment of evolutionary adaptedness (Bennett 2020). For most of human evolutionary history our ancestors
practiced a foraging subsistence characterized by high physical outdoor activity, a diet
rich in fiber and vegetable food with a low content of sugar and fat, an extremely low rate
of overweight and obesity (Irons 1998) and a low
chronic stress level. Therefore, the increasing rates of headache disorders may be due to
the fact, that we are now living in an environment to which we are not adapted. Headaches
may be one result of a mismatch between our evolutionary heritage and our current life
circumstances. This has become especially true over the last decades of our daily life when
we adopt a sedentary lifestyle typically characterized by spending a lot of time sitting in
front of computer or TV screens, low physical outdoor activity, overnutrition with high fat
and sugar intake, smoking, sleeplessness, a chronic Vitamin D deficiency, and a high chronic
stress level (Walker et al. 2003).
All these parameters are associated with the occurrence of headaches. One of the most
commonly named trigger factors for primary headaches is chronic stress (Boardman et al. 2005; Rasmussen 1993; Wöber et al. 2006; Wöber and Wöber-Bingöl 2010; Zivadinov et al. 2003). Chronic stress is often associated with sleep
disturbances and sleeplessness. Suffering from sleep disturbances or sleeplessness increases
headache frequency as well as headache intensity. This is true of TTH as well as of migraine
(Pellegrino et al. 2018).
In the present study, we focused on the complex associations between recent lifestyle
patterns and the frequency as well as the intensity of TTH and migraine. Therefore, we
tested the following two hypotheses:
| 1. | Recent lifestyle, characterized by low physical activity, little outdoor activity, low
average sleeping hours, a high body mass index, and long periods spent sitting in front
of computer or TV screens is associated with an increased frequency and an increased
intensity of migraine and tension-type headaches. |
| 2. | High chronic stress levels are associated with an increased frequency and an increased
intensity of migraine and tension-type headaches. |
Sample and methods
Study design
Due to the Covid-19 situation, data collection could take place online only. SosciSurvey
(Leiner 2019) was used to distribute a
specially developed questionnaire containing 72 items and the questionnaire of the
“Trierer Inventar zum chronischen Stress“ (Trier Inventory of Chronic Stress, TICS)
according to Schulz et al. (Schulz et al. 2004).
The link to the questionnaire was posted in several self-help groups for migraines or
headaches, and the distribution followed a virtual snowball sampling, using social media
platforms, such as Facebook, WhatsApp, and others. Snowball sampling is a
non-probability sampling technique where existing study participants
distribute the links among their friends and acquaintances. Consequently, the sample size
grows like a rolling snowball (Goodman 1961;
Baltar and Brunet 2012). The questionnaire was
accessible online from the 21st December 2020 to the 21st February
2021.
For inclusion in the analyses, the following criteria were defined: female sex, aged
between 18 and 65 years, and diagnosis of migraine or TTH. In addition, the following
strict exclusion criteria were defined: age younger than 18 years or older than 65 years,
chronic or acute diseases which might affect headache, any kind of medication which might
induce headache.
Participants
In total, 219 people filled out the survey completely, but only 173 women aged between 18
and 65 years (x=34.8 years, SD=12.7 years) fulfilled the
strict inclusion criteria mentioned above. Therefore, 35 male participants, 3 participants
who did not identify as male or female, one woman who did not agree to the data usage for
the analysis, one woman older than 65 years, and one woman whose health condition, might
have influenced the intensity or frequency of headache validity for the analysis were
excluded. Although we have no information about the birth places of the participants, all
participants lived in German speaking countries and were able to understand and fill out a
complex questionnaire in German language.
Questionnaire
A 72-item questionnaire was developed for this study. The questionnaire consisted of
items concerning sociodemographic characteristics, questions specific to migraine and TTH,
and questions about lifestyle patterns.
Sociodemographic questions included, for example, age, height, body weight, work, and
education. For the questions regarding migraines as well as TTHs, the “International
classification of headache diseases ICHD-3” by the Headache Classification Committee of
the International Headache Society (Headache
Classification Committee of the International Headache Society 2018) was used.
The classifications for migraine and TTH and their respective types were used to group the
participants according to their headache type or types. The first question described the
particular headache and asked the participants whether or not they had experienced it.
Those who chose the “yes” or the “not sure” option were then given several more questions
regarding the respective headache type. This was done for migraine-type headaches and
TTHs. This approach was chosen, so that participants who did not know what classifies as a
migraine or a TTH, were able to answer according to the description.
Later on, all participants were asked some extra questions about headaches. Factors that
cause or worsen headaches as well as those helping with improving headaches were also
covered in the survey.
The questions regarding lifestyle included topics like screen time, physical activity
patterns, sleep quality, time spent outdoors, and smoking. Furthermore, a section
addressing the impact of menstrual cycle on headaches was included. Since no significant
interaction between menstrual cycle parameters and headaches could be proven, menstrual
cycle characteristics were not considered in further analyses.
Trier Inventory of Chronic Stress
The “Trierer Inventar zum chronischen Stress” (Trier Inventory of Chronic Stress, TICS)
according to Schulz et al. (Schulz et al. 2004)
was used to determine the chronic stress levels of the participants. The TICS consists of
57 questions and is a standardized questionnaire, providing reliability. The 57 questions
are divided into different categories: work overload, social overload, pressure to
perform, work discontent, excessive demands from work, lack of social recognition, social
tensions, social isolation, and chronic worrying and a screening scale for chronic stress.
Each question item is rated on a scale from “never” 0 to “very often” 4. The values of the
questions for the different categories are then summed. The sums are then translated to
T-scores according to the manual.
Statistical Analysis
The statistical analysis was conducted with IBM SPSS Statistics 27. All parameters were
tested for normal distribution by using Kolmogorov-Smirnov tests. Since no normal
distribution could be verified for all metric variables, exclusively non-parametric
procedures were applied. Chi-square tests, Fisher-Exact-tests and Fisher-Halton-Freeman
tests were used for the analysis. Chi-square-tests were preferred, but Fisher-Exact-tests
and Fisher-Halton-Freeman tests were applied when more than 20% of the cell count was
smaller than 5. Group differences were tested by using Kruskal-Wallis-H-tests. A power
analysis was computed to test whether the small sample size allowed spearman rank
correlations and multiple regression analyses. Spearman correlations were computed to test
the correlation patterns between headache characteristics and lifestyle factors as well as
chronic stress parameters with respect to their statistical significance. Multiple
regression analyses were computed to test association patterns between headache
characteristics and lifestyle parameters as well as chronic stress levels. The
significance limit was set at p < 0.05 for all tests.
Results
Sample description
The participants were assigned to four groups according to the characteristics of their
headaches. 41 women suffered from migraine, 20 women suffered from TTH, 97 women suffered
from migraine and TTH, 19 women did not suffer from headaches. The mean age of the four
subgroups (no headache, migraine, TTH and TTH + migraine) differed significantly
(p=0.003). Women without headache showed the lowest mean age (x=26.6
years ± 5.6), while women suffering from migraine (x=38.0 years ± 13.8) represented the
oldest group. The mean age of women suffering from TTH was 31.2 years ± 14.3, while women
suffering from TTH and migraine were on average 36.3 years ± 12.6 old. Age correlated
significantly with the frequency of migraine attacks among women suffering from migraine
only (p=0.040) and among women suffering from migraine and TTH
(p=0.004). Table 1 presents
the socioeconomic characteristics and the weight status of the participants for each
subgroup separately. Neither socioeconomic characteristics nor weight status differed
significantly between the four subgroups.
Table 1 Sample description socioeconomic parameters according to headache
group
|
migraine |
tension-type headaches |
migraine + tension-type headaches |
no headache |
| n |
41 |
20 |
97 |
19 |
| Civil status |
| single |
26.8% |
45% |
29.9% |
38.9% |
| married/cohabitation |
73.2% |
55% |
69% |
61.1% |
| divorced/separated |
0.0% |
0 |
1% |
0.0% |
| Educational level |
| compulsory education |
0% |
0% |
2.1% |
10.5% |
| vocational training |
14.6% |
0% |
11.5% |
0% |
| high school |
46.3% |
40% |
36.4% |
36.8% |
| University degree |
36.6% |
60% |
50.0% |
52.7% |
| Occupation |
| fulltime |
41.5% |
10.0% |
28.9% |
21.1% |
| part-time |
34.1% |
40.0% |
33.0% |
52.6% |
| unemployed |
2.4% |
5.0% |
7.3% |
5.3% |
| student |
22.0% |
70,0% |
36.1% |
73.7% |
| retired |
7.3% |
10.0% |
10.3% |
0.0% |
| Children |
| yes |
51.2% |
20 |
25.8% |
22.2% |
| no |
48.8% |
80 |
74.2% |
77.8% |
| Smoking |
| yes |
14.6% |
25.0% |
12.4% |
21.1% |
| no |
85.4% |
75.0% |
87.6% |
78.9% |
| Weight status (BMI) |
| <18.50kg/m2 |
7.7% |
5.3% |
9.7% |
15.8% |
| 18.50-24.99kg/m2 |
53.8% |
68.4% |
66.7% |
68.4% |
| 25.00-29.99kg/m2 |
24.4% |
21.1% |
12.9% |
10.5% |
| ≥30.00kg/m2 |
12.8% |
5.3% |
10.8% |
5.3% |
Factors which might trigger migraine or tension-type headaches
An extensive list of possible migraine or TTH triggers was provided. The most often
chosen trigger factors for migraine as well as TTH were change in weather, stress, lack of
sleep, and neck and muscle tension (figure 1).
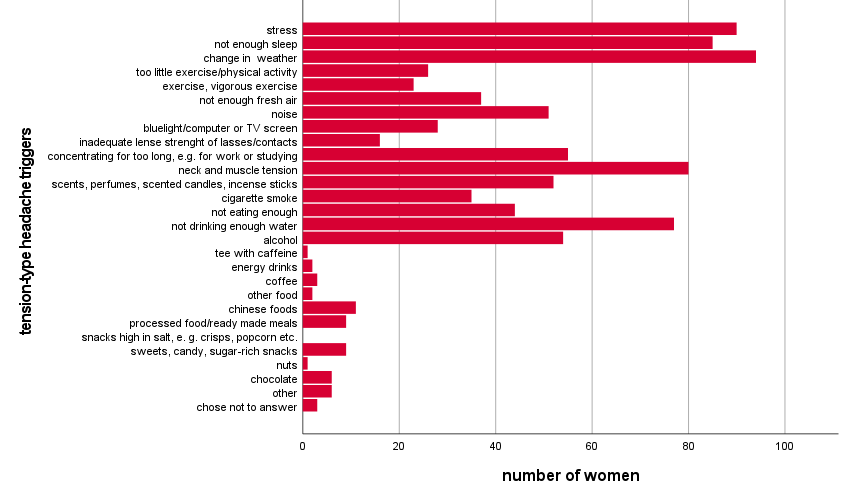
Figure 1 Trigger factors of tension-type headache attacks
Lifestyle parameters and headache characteristics
The lifestyle parameters of the participants are presented in Table 2. The four subgroups did not differ significantly in the
lifestyle parameters.
Table 2 Sample description lifestyle parameters according to headache
group
|
Migraine |
TTH |
migraine + TTH |
no headache |
| N |
41 |
20 |
97 |
19 |
| Screen time (hours/ day) |
| <1 hour |
2.5% |
0.0% |
1.0% |
0.0% |
| 1-6 hours |
47.5% |
55.0% |
58.3% |
66.7% |
| 6-12 hours |
50.0% |
45.0% |
39.6% |
33.3% |
| >12 hours |
0.0% |
0.0% |
1.0% |
0.0% |
| Outdoor activity (hours/week
) |
| <4 hours |
48.8% |
50.0% |
32.0% |
33.3% |
| 4-7 hours |
36.6% |
40.0% |
41.2% |
33.3% |
| 7-14 hours |
7.3% |
5.0% |
18.6% |
27.8% |
| >14 hours |
7.3% |
5.0% |
8.2% |
5.6% |
| Physical exercise
(hours/week) |
| No exercise |
10.5% |
15.8% |
11.5% |
26.3% |
| <2 hours |
23.7% |
42.1% |
28.1% |
15.8% |
| 2-4 hours |
36.8% |
15.8% |
26.0% |
36.8% |
| 4-7 hours |
17.1% |
21.1% |
28.1% |
15.8% |
| 7-9 hours |
4.9% |
0.0% |
5.2% |
0.0% |
| <9 hours |
4.9% |
5.3% |
1.0% |
5.3% |
| Sleep (hours/day) |
| <6 hours |
22.0% |
20.0% |
19.8% |
27.8% |
| 7-8 hours |
70.7% |
70.0% |
68.8% |
50.0% |
| >9 hours |
7.3% |
10.0% |
11.5% |
22.2% |
Within the subgroups however, the frequency of headache attacks correlated significantly
with some lifestyle parameters. In detail, migraine frequency correlated significantly
positively (p=0.004) with the hours spent in front of a computer screen
per day. The frequency of TTH correlated significantly negatively with the sleeping hours
per day (p=0.010) and significantly negatively with the hours of physical
exercise per week (p=0.047). However, we have to be aware, that, with the
exception of the correlation between hours of physical exercise per week and frequency of
TTH, the correlation coefficients are below 0.30 and therefore explain less than 9% of
variation. (Table 3).
Table 3 Headache patterns and lifestyle factors. Spearman
correlations.
|
migraine |
tension-type headache |
|
frequency |
Intensity |
frequency |
intensity |
| Lifestyle parameters |
M +TTH |
M |
M +TTH |
M |
TTH +M |
TTH |
TTH + M |
TTH |
| Body mass index
(kg/m2) |
0.12 |
-0.05 |
-0.07 |
-0.11 |
-0.08 |
0.42 |
-0.07 |
0.19 |
| Sleep (hours/ day) |
-0.07 |
-0.08 |
-0.16 |
-0.24 |
-0.29** |
0.21 |
0.17 |
0.44 |
| Screen time (hours/day) |
0.05 |
0.29** |
-0.08 |
0.01 |
-0.11 |
0.22 |
0.17 |
0.37 |
| Outdoor activity (hours/week) |
0.13 |
0.05 |
0.03 |
-0.23 |
-0.08 |
-0.33 |
0.17 |
-0.19 |
| Exercise (hours/week) |
-0.07 |
0.19 |
0.11 |
-0.07 |
0.01 |
-0.48* |
-0.15 |
-0.17 |
These results were corroborated partly by the multiple regression analyses. While
migraine frequency was significantly negatively (p=0.011) associated with
age only, and migraine intensity showed no significant associations with any lifestyle
parameter nor with age, the frequency of TTH tension-type headache attacks was
significantly negatively associated with the sleeping hours per day
(p=0.029). The intensity of TTH was significantly positively related to
smoking (p=0.049), the time spent in front of computer screens per day
(p=0.049), and subjective stress level (p=0.026). The
sleeping hours per day, however, were significantly negatively (p=0.009)
associated with the intensity of TTH. The r-squares were quite low (Table 4).
Table 4 The impact of lifestyle parameters on the intensity and frequency of migraine and
TTH headache. Multiple regression analysis.
| |
R2 |
B |
Sig |
95% CI |
R2 |
B |
Sig |
95% CI |
|
|
Migraine
frequency |
|
Migraine
intensity |
| Age |
0.28 |
-0.02 |
0.011 |
-0.03 – -0.01 |
0.27 |
0.01 |
0.071 |
-0.01 – 0.02 |
| Smoking |
|
0.04 |
0.875 |
-0.45 – 0.53 |
|
0.04 |
0.776 |
-0.25 – 0.33 |
| Screen time
(hours/day) |
|
0.11 |
0.510 |
-0.21 – 0.42 |
|
0.02 |
0.837 |
-0.16 – 0.20 |
| Exercise
(hours/week) |
|
0.05 |
0.503 |
-0.11 – 0.21 |
|
0.04 |
0.426 |
-0.05 – 0.13 |
| Outdoor activity
(hours/week) |
|
0.02 |
0.848 |
-0.17 – 0.21 |
|
-0.03 |
0.620 |
-0.08 – 0.13 |
| Sleep
(hours/day) |
|
-0.15 |
0.350 |
-0.45 – 0.16 |
|
-0.07 |
0.446 |
-0.25 – 0.11 |
| Body mass index
(kg/m2) |
|
0.03 |
0.173 |
-0.01 – 0.06 |
|
0.01 |
0.782 |
-0.02 – 0.02 |
|
TTH frequency |
TTH intensity |
| Age |
0.36 |
0.07 |
0.228 |
-0.05 – 0.19 |
0.45 |
0.01 |
0.805 |
-0.01 – 0.01 |
| Smoking |
|
2.67 |
0.177 |
-1.23 – 6.57 |
|
0.36 |
0.049 |
0.01 – 0.73 |
| Screen time
(hours/day) |
|
2.05 |
0.157 |
0.80 – 4.90 |
|
0.25 |
0.049 |
0.01 – 0.51 |
| Exercise
(hours/week) |
|
-0.81 |
0.265 |
-2.23 – 0.62 |
|
-0.03 |
0.614 |
-0.16 – 0.09 |
| Outdoor activity
(hours/week) |
|
-0.83 |
0.296 |
-2.41 – 0.74 |
|
-0.14 |
0.067 |
-0.28 – 0.01 |
| Sleep
(hours/day) |
|
-3.11 |
0.029 |
-5.88 – -0.33 |
|
-0.31 |
0.009 |
-0.53 – -0.08 |
| Body mass index
(kg/m2) |
|
-0.02 |
0.901 |
-0.35 – 0.31 |
|
0.01 |
0.808 |
-0.03 – 0.03 |
Chronic stress and headache characteristics
Chronic stress was estimated by using the Trier Inventory of chronic stress (TICS). The
four subgroups differed significantly only in the component social overload
(p=0.045). The highest scores for the components social overload and
pressure to perform were found for women suffering from TTH and migraine, while the
highest scores for work overload, lack of social recognition and social tensions were
found in women suffering from migraine only. Women affected by TTH only showed the highest
scores for work discontent, excessive demands from work, social isolation, and chronic
worrying. Women without headache always showed the lowest scores of chronic stress
components.
Table 5 Headache patterns and stress factors. Spearman
correlations.
|
migraine |
tension-type headache |
|
frequency |
intensity |
frequency |
intensity |
|
M +TTH |
M |
M+TTH |
M |
TTH +M |
TTH |
TTH + M |
TTH |
| Work overload |
-0.05 |
0.12 |
-0.04 |
-0.11 |
-0.01 |
0.38 |
0.26** |
0.28 |
| Social overload |
-0.09 |
0.12 |
-0.16 |
-0.02 |
0.01 |
-0.14 |
0.11 |
0.19 |
| Pressure to perform |
0.01 |
0.17 |
-0.03 |
-0.29* |
-0.13 |
0.19 |
0.18 |
0.19 |
| Work discontent |
-0.04 |
0.31 * |
-0.05 |
-0.17 |
-0.11 |
0.35 |
0.12 |
0.21 |
| Excessive work demands |
-0.16 |
0.09 |
-0.15 |
-0.04 |
-0.09 |
0.41 |
0.09 |
0.39 |
| Lack of social recognition |
-0.09 |
0.31* |
0.07 |
-0.01 |
0.02 |
0.48* |
-0.02 |
0.47* |
| Social tensions |
-0.04 |
-0.08 |
0.19* |
-0.14 |
-0.13 |
0.29 |
0.07 |
0.49* |
| Social isolation |
0.17* |
0.09 |
-0.09 |
-0.09 |
0.28* |
0.05 |
0.02 |
0.26 |
| Chronic worrying |
0.03 |
0.11 |
0.18* |
-0.12 |
-0.03 |
0.53* |
0.23* |
0.25 |
| chronic stress |
-0.11 |
0.16 |
0.07 |
-0.0 |
-0.01 |
0.37 |
0.21* |
0.20 |
As presented in Table 5, the frequency of
migraine attacks correlated significantly positively with work discontent
(p=0.026), a lack of social recognition (p=0.030), and
social isolation (p=0.046). The intensity of migraine correlated
significantly positively with pressure to perform (p=0.044), social
tensions (p=0.040), and chronic worrying (p=0.047). The
frequency of TTH correlated significantly with a lack of social recognition
(p=0.049), social isolation (p=0.016), and chronic
worrying (p=0.028). The intensity of TTH correlated significantly
positively with work overload (p=0.011), a lack of social recognition
(p=0.041), social tensions (p=0.031), and chronic
worrying (p=0.024). As pointed out above, the correlation coefficients
were quite low - mostly below 0.30. Therefore, we have to state that even some of the
statistically significant correlations may only indicate a low biological importance.
The results of the multiple regression analyses are presented in Table 6.
Table 6 The impact of chronic stress on the intensity and frequency of migraine and TTH
headache
| |
R2 |
B |
Sig |
95% CI |
R2 |
B |
Sig |
95% CI |
|
migraine
frequency |
migraine
intensity |
| Work overload |
0.26 |
0.01 |
0.966 |
-0.02 – 0.02 |
0.44 |
-0.01 |
0.509 |
-0.02 – 0.01 |
| Social overload |
|
-0.01 |
0.697 |
-0.02 – 0.02 |
|
0.01 |
0.088 |
-0.01 – 0.02 |
| Pressure to perform |
|
0.02 |
0.222 |
-0.01 – 0.04 |
|
-0.02 |
0.005 |
-0.03 - -0.01 |
| Work discontent |
|
0.01 |
0.984 |
-0.02 – 0.02 |
|
-0.01 |
0.233 |
-0.02 – 0.01 |
| excessive work demands |
|
-0.01 |
0.813 |
-0.02 – 0.02 |
|
-0.01 |
0.093 |
-0.02 – 0.01 |
| Lack of social recognition |
|
0.01 |
0.390 |
-0.01 – 0.03 |
|
0.01 |
0.966 |
-0.01 – 0.01 |
| Social tensions |
|
-0.01 |
0.191 |
-0.03 – 0.01 |
|
0.01 |
0.480 |
-0.01 – 0.01 |
| Social isolation |
|
0.01 |
0.123 |
-0.03 – 0.03 |
|
0.01 |
0.992 |
-0.01 – 0.01 |
| Chronic worrying |
|
0.02 |
0.160 |
-0.01 – 0.05 |
|
0.01 |
0.163 |
-0.01 – 0.03 |
| chronic stress |
|
-0.03 |
0.282 |
-0.08 – 0.02 |
|
0.01 |
0.835 |
-0.02 – 0.03 |
|
TTH frequency |
TTH intensity |
| Work overload |
0.38 |
0.03 |
0.758 |
-0.16 – 0.22 |
0.31 |
0.01 |
0.112 |
-0.01 – 0.03 |
| Social overload |
|
-0.04 |
0.680 |
-0.22 – 0.14 |
|
-0.01 |
0.898 |
-0.02 – 0.01 |
| Pressure to perform |
|
-0.03 |
0.796 |
-0.23 – 0.18 |
|
0.01 |
0.433 |
-0.01 – 0.03 |
| Work discontent |
|
-0.03 |
0.671 |
-0.19 – 0.12 |
|
0.01 |
0.346 |
-0.01 – 0.02 |
| excessive work demands |
|
-0.16 |
0.141 |
-0.37 – 0.05 |
|
-0.01 |
0.798 |
-0.02 – 0.02 |
| Lack of social recognition |
|
0.19 |
0.037 |
0.01 – 0.37 |
|
-0.01 |
0.242 |
-0.03 – 0.01 |
| Social tensions |
|
-0.09 |
0.253 |
-0.23 – 0.06 |
|
0.01 |
0.454 |
-0.01 – 0.02 |
| Social isolation |
|
0.14 |
0.021 |
0.02 – 0.26 |
|
0.01 |
0.798 |
-0.01 – 0.02 |
| Chronic worrying |
|
0.01 |
0.961 |
-0.29 – 0.31 |
|
0.01 |
0.418 |
-0.02 – 0.04 |
| chronic stress |
|
0.20 |
0.350 |
-0.23 – 0.63 |
|
0.01 |
0.642 |
-0.03 – 0.05 |
While the pressure to perform was positively associated with migraine intensity
(p=0.005), a lack of social recognition (p=0.037) and
social isolation (p=0.021) were significantly positively associated with
the frequency of TTH. Again, the r-squares were quite low (below 0.44).
Discussion
It was hypothesized that recent lifestyle characterized by low physical activity, little
outdoor activity, low average sleeping hours, a high body mass index, and long periods of
time spent sitting in front of computer or TV screens is associated with an increased
frequency and an increased intensity of migraine and TTH. Furthermore, it was tested,
whether high chronic stress levels are associated with an increased frequency, and an
increased intensity of migraine and TTH.
Both hypotheses could be verified in the present study. This means that recent lifestyle
factors as well as the burden of chronic stress trigger the intensity and frequency of
primary headaches. However, before we start to discuss the results in detail, we have to
state that this study has some important limitations. On the one hand, the sample size was
quite small – only 173 adult women were included in the study and the three headache
subgroups were quite small. Although the power analyses allow Spearman rank correlations and
multiple regression analyses for such small group sizes, we have to state, that this study
has the character of a pilot study. Furthermore, and although few correlations were of
statistical significance, the rather low correlation coefficients below 0.30 point to low
biological importance of these associations. This is also true of the rather low r-squares
found during the multiple regression analyses. Therefore, we have to be aware that even
though a statistical significance could be observed, the biological importance is only
modest. Another limitation is, that data collection took place using an online survey only.
This study design is due to the Covid-19 pandemic, which made face-to-face interviews and
direct data collection impossible. It was therefore not possible to include the effect of
Vitamin D deficiency on headaches (Kjaergaard et al.
2012), because it was not possible to collect blood samples of the participants.
In our sample, 23.7% of the participants suffered from migraine, 11.6% from TTH, but 54.3%
suffered from both headache conditions. We compared the three headache subgroups and one
group of women who reported to be not affected by headaches. The four groups did not differ
significantly in sociodemographic factors, with the exception of age. The effects of
socioeconomic parameters have been widely described (Molarius et al. 2008). Furthermore, the well-described effects of menstrual cycle
patterns as trigger factors for headaches (Arjona et al.
2007; Campelo et al. 2021) could not be
proven in the present study. Women suffering from different types of primary headaches and
women without headaches did not differ significantly in lifestyle parameters such as weight
status, nicotine consumption, sleeping hours per day, physical exercise hours per week,
outdoor activity per week, or hours spent in front of a computer or TV screen per day.
Furthermore, the four groups did not differ significantly in the components of chronic
stress, with the exception of social overload. Consequently, no significant differences in
lifestyle parameters and in most of the chronic stress components could be observed between
the four subgroups. Both, women suffering from TTH as well as women suffering from migraine
attacks reported changes in weather, stress, a lack of sleep and neck or muscle tension as
the most important trigger factors of headache. These observations are in accordance with
the results of several other studies (Marrelli et al.
1988; Altura and Altura 2001; Engstrøm et al. 2014b; Meyer et al. 2016; Rafique et al.
2020; Engstrøm et al. 2014a).
Without any doubt, sleep is important for general health and quality of life, because sleep
is required to regenerate, relax and revitalize, and it is needed to recharge emotional
batteries (Mukherjee et al. 2015; Ohlmann and O'Sullivan 2009). In the present study, a
significant association between a lack of sleeping hours per day and increased frequency and
intensity of TTH could be observed. The direction of causality cannot be determined, that
is, it is possible that a lack of sleep increases TTH attacks or the headaches might cause
the lack of sleep. Nevertheless, these findings are partly in accordance with previous
studies. Kelman and Rains (Kelman and Rains 2005)
found that participants who slept less, in particular less than six hours per night,
experienced more frequent and severe headaches. Correspondingly, Alberti (Alberti 2006) detected that short-time sleepers had more
frequent headaches than long-time sleepers. Furthermore, no significant associations between
sleeping hours and migraine frequency and intensity were detected. These findings are in
contrast to the findings of Ødegård et al. (Ødegård et al.
2010) and Walters et al. (Walters et al.
2014) who reported a significant association between poor sleep quality as well as
sleep disturbances and migraine frequency. Some studies show that migraine attacks can also
be provoked by excessive sleep (Kelman and Rains
2005; Rafique et al. 2020; Wöber and Wöber-Bingöl 2010).
Sleep is only one factor which affects headache. Our daily lifestyle is often characterized
by a low rate of physical exercise and/or outdoor activity. In the present study, however,
no significant associations between the amount of physical activity per week and migraine
attacks occurred. Similar results have been provided by Rasmussen (Rasmussen 1993) and Winter et al. (Winter et al. 2011), who also did not find any connections of migraine with
physical activity patterns. TTH frequency showed a significant negative association with the
number of exercise hours per week, while no significant associations between headache
characteristics and outdoor activity were found. These findings are in contrast to the
results of (Hagen et al. 2018), who reported no
significant associations between physical activity and the frequency and intensity of TTH.
In our study, women with strong and very strong pain intensity spent less hours per week on
exercise. This result is in accordance to (Kikuchi et al.
2007), who showed that greater intensities led to less physical activity. On the
other hand, the present study yielded a significantly positive association between TTH
intensity and hours spent in front of a computer or TV screen per day. Consequently, a
sedentary lifestyle seems to trigger TTH pain intensity, although the headaches may be
triggered by looking at the screen per se.
Another well-documented trigger of headache is chronic stress (Boardman et al. 2005; Pellegrino
et al. 2018; Rasmussen 1993; Wöber et al. 2006; Wöber and Wöber-Bingöl 2010; Zivadinov et al.
2003). In this study, stress was measured by using the Trier Inventory for Chronic
Stress (TICS) (Schulz et al. 2004). Headache
sufferers generally had higher TICS- scores than the non-headache controls. These
differences, however, were not of statistical significance. Migraine frequency and intensity
correlated with various components of chronic stress, such as pressure to perform, work
discontent, lack of social recognition, social tensions, social isolation and chronic
worrying. This was also true of TTH for which, in addition, a significant association with
work overload was found. In general, the higher the chronic stress scores, the higher were
the frequency and intensity of migraine and TTH. However, these associations were only found
for correlations using multiple regression models and only the pressure to perform score was
significantly associated with migraine intensity. Similarly, Antonov and Isacson (Antonov and Isacson 1997) found work overload to be
significantly associated with the intensity of TTH. Connections of work stress and headaches
have also been made by Lynberg et al. (Lyngberg et al.
2005).
A possible explanation for links of chronic stress and TTH is that stress can lead to
increased pain sensitivity and can affect pain processing. Abnormal pain processing is also
relevant in TTH (Cathcart et al. 2010; Cathcart et al. 2012). Additionally, stress often
causes jaw clenching and contractions of muscles in the neck. This could also have effects
on headaches (Cathcart et al. 2010; Cathcart et al. 2012). Another aspect is that chronic
stress can cause changes in the brain and cause inability to remain in allostasis (Maleki et al. 2012; Pellegrino et al. 2018). This could cause and worsen headaches. Summing up, this
study mainly found connections of stress with TTH, in particular with the intensity of them.
In accordance to this, several other studies found associations too and several explanations
for possible connecting mechanisms exist.
The results of the present study emphasise the effect of lifestyle parameters and chronic
stress components on primary headache characteristics. These association patterns are of
importance, because primary headache disorders have a high global prevalence and pose a
burden for many individuals. From the viewpoint of Evolutionary Anthropology, we have to be
aware that modern environments and recent lifestyle patterns differ drastically from those
experienced by our ancestors during human evolution and humankind’s past. This change has
led to so called lifestyle and environment mismatches, which have been linked to several
health issues. Common mismatches are lack of physical activity, high caloric intake, and
high levels of psychological stress, bright lights etc. Lifestyle factors also have the
potential to be associated with headache disorders, such as migraines and TTH. Sensitivity
to environmental inputs might have posed an evolutionary advantage. Many migraine triggers
are potentially dangerous in high doses. Thus, detecting them in smaller doses could have
been advantageous. Triggers like that are e.g., scents and odours, foods, but also stress
and other factors. Additionally, the mismatch in lifestyle could also have had impacts on
headache disorders as well as caused a rise in prevalence (Bennett 2020; Brenner et al. 2015; Loder 2002).
We can conclude that the increasing prevalence of primary headache disorders may be
triggered by recent lifestyle characteristics which are in marked contrast to the lifestyle
we are adapted for.
References
Alberti, A. (2006). Headache and sleep. Sleep
Medicine Reviews 10 (6), 431–437. https://doi.org/10.1016/j.smrv.2006.03.003.
Altura, B. M./Altura, B. T. (2001). Tension
headaches and muscle tension: is there a role for magnesium? Medical Hypotheses 57 (6),
705–713. https://doi.org/10.1054/mehy.2001.1439.
Antonov, K./Isacson, D. (1997). Headache in
Sweden: the importance of working conditions. Headache 37 (4), 228–234. https://doi.org/10.1046/j.1526-4610.1997.3704228.x.
Arjona, A./Rubi-Callejon, J./Guardado-Santervas,
P./Serrano-Castro, P./Olivares, J. (2007). Menstrual tension-type headache: evidence for
its existence. Headache 47 (1), 100–103. https://doi.org/10.1111/j.1526-4610.2007.00656.x.
Baltar, F./Brunet, I. (2012). Social research 2.0:
virtual snowball sampling method using Facebook. Internet Research 22 (1), 57–74.
https://doi.org/10.1108/10662241211199960.
Bennett, K. (2020). Environment of evolutionary
adaptedness (EEA). In: V. Zeigler-Hill/T. K. Shackelford (Eds.). Encyclopedia of
personality and individual differences. Cham, Springer International Publishing,
1388–1391.
Boardman, H. F./Thomas, E./Millson, D. S./Croft,
P. R. (2005). Psychological, sleep, lifestyle, and comorbid associations with headache.
Headache 45 (6), 657–669. https://doi.org/10.1111/j.1526-4610.2005.05133.x.
Brenner, S. L./Jones, J. P./Rutanen-Whaley, R.
H./Parker, W./Flinn, M. V./Muehlenbein, M. P. (2015). Evolutionary mismatch and chronic
psychological stress. Journal of Evolutionary Medicine 3, 1–11. https://doi.org/10.4303/jem/235885.
Campelo, A. G. D./Lima, D. A./Britto, G. R.
C./Moraes, I. S. L./Almeida, R. M./Silva-Néto, R. P. (2021). Does menstruation-related
headache occur exclusively in women with migraine? Acta Neurologica Belgica 121 (4),
1035–1038. https://doi.org/10.1007/s13760-021-01646-w.
Cathcart, S./Bhullar, N./Immink, M./Della Vedova,
C./Hayball, J. (2012). Pain sensitivity mediates the relationship between stress and
headache intensity in chronic tension-type headache. Pain Research & Management 17
(6), 377–380. https://doi.org/10.1155/2012/132830.
Cathcart, S./Winefield, A. H./Lushington,
K./Rolan, P. (2010). Stress and tension-type headache mechanisms. Cephalalgia 30 (10),
1250–1267. https://doi.org/10.1177/0333102410362927.
Engstrøm, M./Hagen, K./Bjørk, M. H./Stovner, L.
J./Sand, T. (2014a). Sleep quality and arousal in migraine and tension-type headache:
the headache-sleep study. Acta Neurologica Scandinavica. Supplementum (198), 47–54.
https://doi.org/10.1111/ane.12237.
Engstrøm, M./Hagen, K./Bjørk, M./Stovner, L.
J./Stjern, M./Sand, T. (2014b). Sleep quality, arousal and pain thresholds in
tension-type headache: a blinded controlled polysomnographic study. Cephalalgia 34 (6),
455–463. https://doi.org/10.1177/0333102413515339.
Goodman, L. A. (1961). Snowball Sampling. The
Annals of Mathematical Statistics 32 (1), 148–170. https://doi.org/10.1214/aoms/1177705148.
Hagen, K./Åsberg, A. N./Stovner, L./Linde,
M./Zwart, J.-A./Winsvold, B. S./Heuch, I. (2018). Lifestyle factors and risk of migraine
and tension-type headache. Follow-up data from the Nord-Trøndelag Health Surveys
1995-1997 and 2006-2008. Cephalalgia 38 (13), 1919–1926. https://doi.org/10.1177/0333102418764888.
Hamelsky, S. W./Lipton, R. B./Stewart, W. F.
(2005). An assessment of the burden of migraine using the willingness to pay model.
Cephalalgia 25 (2), 87–100. https://doi.org/10.1111/j.1468-2982.2005.00797.x.
Headache Classification Committee of the
International Headache Society (2018). The international classification of headache
disorders, 3rd edition. Cephalalgia 38 (1), 1–211. https://doi.org/10.1177/0333102417738202.
Irons, W. (1998). Adaptively relevant environments
versus the environment of evolutionary adaptedness. Evolutionary Anthropology: Issues,
News, and Reviews 6 (6), 194–204. https://doi.org/10.1002/(SICI)1520-6505(1998)6:6<194::AID-EVAN2>3.0.CO;2-B.
Jensen, R./Stovner, L. J. (2008). Epidemiology and
comorbidity of headache. The Lancet Neurology 7 (4), 354–361. https://doi.org/10.1016/S1474-4422(08)70062-0.
Kelman, L./Rains, J. C. (2005). Headache and
sleep: examination of sleep patterns and complaints in a large clinical sample of
migraineurs. Headache 45 (7), 904–910. https://doi.org/10.1111/j.1526-4610.2005.05159.x.
Kikuchi, H./Yoshiuchi, K./Ohashi, K./Yamamoto,
Y./Akabayashi, A. (2007). Tension-type headache and physical activity: an actigraphic
study. Cephalalgia 27 (11), 1236–1243. https://doi.org/10.1111/j.1468-2982.2007.01436.x.
Kjaergaard, M./Eggen, A. E./Mathiesen, E.
B./Jorde, R. (2012). Association between headache and serum 25-hydroxyvitamin D: the
Tromsø Study: Tromsø 6. Headache 52 (10), 1499–1505. https://doi.org/10.1111/j.1526-4610.2012.02250.x.
Leiner, D. J. (2019). SoSci Survey. Version
3.1.06.
Linde, M./Dahlöf, C. (2004). Attitudes and burden
of disease among self-considered migraineurs: a nation-wide population-based survey in
Sweden. Cephalalgia 24 (6), 455–465. https://doi.org/10.1111/j.1468-2982.2004.00703.x.
Lipton, R. B./Bigal, M. E./Kolodner, K./Stewart,
W. F./Liberman, J. N./Steiner, T. J. (2003). The family impact of migraine:
population-based studies in the USA and UK. Cephalalgia 23 (6), 429–440. https://doi.org/10.1046/j.1468-2982.2003.00543.x.
Lipton, R. B./Hamelsky, S. W./Kolodner, K.
B./Steiner, T. J./Stewart, W. F. (2000). Migraine, quality of life, and depression: a
population-based case-control study. Neurology 55 (5), 629–635. https://doi.org/10.1212/wnl.55.5.629.
Loder, E. (2002). What is the evolutionary
advantage of migraine? Cephalalgia 22 (8), 624–632. https://doi.org/10.1046/j.1468-2982.2002.00437.x.
Lyngberg, A. C./Rasmussen, B. K./Jørgensen,
T./Jensen, R. (2005). Incidence of primary headache: a Danish epidemiologic follow-up
study. American Journal of Epidemiology 161 (11), 1066–1073. https://doi.org/10.1093/aje/kwi139.
Maleki, N./Becerra, L./Borsook, D. (2012).
Migraine: maladaptive brain responses to stress. Headache 52 Suppl 2, 102–106. https://doi.org/10.1111/j.1526-4610.2012.02241.x.
Marrelli, A./Marini, C./Prencipe, M. (1988).
Seasonal and meteorological factors in primary headaches. Headache 28 (2), 111–113.
https://doi.org/10.1111/j.1526-4610.1988.hed2802111.x.
Meyer, B./Keller, A./Wöhlbier, H.-G./Overath, C.
H./Müller, B./Kropp, P. (2016). Progressive muscle relaxation reduces migraine frequency
and normalizes amplitudes of contingent negative variation (CNV). The Journal of
Headache and Pain 17, 37. https://doi.org/10.1186/s10194-016-0630-0.
Molarius, A./Tegelberg, A./Ohrvik, J. (2008).
Socio-economic factors, lifestyle, and headache disorders - a population-based study in
Sweden. Headache 48 (10), 1426–1437. https://doi.org/10.1111/j.1526-4610.2008.01178.x.
Mukamal, K. J./Wellenius, G. A./Suh, H.
H./Mittleman, M. A. (2009). Weather and air pollution as triggers of severe headaches.
Neurology 72 (10), 922–927. https://doi.org/10.1212/01.wnl.0000344152.56020.94.
Mukherjee, S./Patel, S. R./Kales, S. N./Ayas, N.
T./Strohl, K. P./Gozal, D./Malhotra, A. (2015). An official American thoracic society
statement: the importance of healthy sleep. recommendations and future priorities.
American Journal of Respiratory and Critical Care Medicine 191 (12), 1450–1458.
https://doi.org/10.1164/rccm.201504-0767ST.
Ødegård, S. S./Engstrøm, M./Sand, T./Stovner, L.
J./Zwart, J.-A./Hagen, K. (2010). Associations between sleep disturbance and primary
headaches: the third Nord-Trøndelag Health Study. The Journal of Headache and Pain 11
(3), 197–206. https://doi.org/10.1007/s10194-010-0201-8.
Ohlmann, K. K./O'Sullivan, M. I. (2009). The costs
of short sleep. AAOHN Journal 57 (9), 381-5; quiz 386-7. https://doi.org/10.3928/08910162-20090817-02.
Pellegrino, A. B. W./Davis-Martin, R. E./Houle, T.
T./Turner, D. P./Smitherman, T. A. (2018). Perceived triggers of primary headache
disorders: A meta-analysis. Cephalalgia 38 (6), 1188–1198. https://doi.org/10.1177/0333102417727535.
Rafique, N./Al-Asoom, L. I./Latif, R./Alsunni, A.
A./Salem, A. M./Alkhalifa, Z. H./Almaharfi, R. M./Alramadan, R. S./Aldajani, Z.
F./Alghadeer, F. A. T./Albaghli, L. A. (2020). Prevalence of Migraine and its
Relationship with Psychological Stress and Sleep Quality in Female University Students
in Saudi Arabia. Journal of pain research 13, 2423–2430. https://doi.org/10.2147/JPR.S270847.
Rasmussen, B. K. (1993). Migraine and tension-type
headache in a general population: precipitating factors, female hormones, sleep pattern
and relation to lifestyle. Pain 53 (1), 65–72. https://doi.org/10.1016/0304-3959(93)90057-V.
Schulz, P./Schlotz, W./Becker, P. (2004). Trierer
Inventar zum Chronischen Stress. Hogrefe.
Steiner, T. J./Stovner, L. J./Katsarava,
Z./Lainez, J. M./Lampl, C./Lantéri-Minet, M./Rastenyte, D./La Ruiz de Torre,
E./Tassorelli, C./Barré, J./Andrée, C. (2014). The impact of headache in Europe:
principal results of the Eurolight project. The Journal of Headache and Pain 15, 31.
https://doi.org/10.1186/1129-2377-15-31.
Stovner, Lj/Hagen, K./Jensen, R./Katsarava,
Z./Lipton, Rb/Scher, Ai/Steiner, Tj/Zwart, J-A (2007). The global burden of headache: a
documentation of headache prevalence and disability worldwide. Cephalalgia 27 (3),
193–210. https://doi.org/10.1111/j.1468-2982.2007.01288.x.
Sutherland, H. G./Albury, C. L./Griffiths, L. R.
(2019). Advances in genetics of migraine. The Journal of Headache and Pain 20 (1), 72.
https://doi.org/10.1186/s10194-019-1017-9.
Tolentino, G. A./Bevilaqua-Grossi, D./Carvalho, G.
F./Carnevalli, A. P. O./Dach, F./Florencio, L. L. (2018). Relationship between headaches
and neck pain characteristics with neck muscle strength. Journal of Manipulative and
Physiological Therapeutics 41 (8), 650–657. https://doi.org/10.1016/j.jmpt.2018.04.003.
van Casteren, D. S./Verhagen, I. E./van der Arend,
B. W. H./van Zwet, E. W./Maassen van den Brink, A./Terwindt, G. M. (2021). Comparing
perimenstrual and nonperimenstrual migraine attacks using an e-diary. Neurology 97 (17),
e1661-e1671. https://doi.org/10.1212/WNL.0000000000012723.
Vos et al./GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369
diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis
for the Global Burden of Disease Study 2019. The Lancet 396 (10258), 1204–1222.
https://doi.org/10.1016/S0140-6736(20)30925-9
Walker, A. R. P./Walker, B. F./Adam, F. (2003).
Nutrition, diet, physical activity, smoking, and longevity. Nutrition 19 (2), 169–173.
https://doi.org/10.1016/s0899-9007(02)00948-6.
Walters, A. B./Hamer, J. D./Smitherman, T. A.
(2014). Sleep disturbance and affective comorbidity among episodic migraineurs. Headache
54 (1), 116–124. https://doi.org/10.1111/head.12168.
Winter, A. C./Hoffmann, W./Meisinger, C./Evers,
S./Vennemann, M./Pfaffenrath, V./Fendrich, K./Baumeister, S. E./Kurth, T./Berger, K.
(2011). Association between lifestyle factors and headache. The Journal of Headache and
Pain 12 (2), 147–155. https://doi.org/10.1007/s10194-010-0286-0.
Wöber, C./Holzhammer, J./Zeitlhofer, J./Wessely,
P./Wöber-Bingöl, C. (2006). Trigger factors of migraine and tension-type headache:
experience and knowledge of the patients. The Journal of Headache and Pain 7 (4),
188–195. https://doi.org/10.1007/s10194-006-0305-3.
Wöber, C./Wöber-Bingöl, Ç. (2010). Triggers of
migraine and tension-type headache. In: Nappi G./M. A. Muskowitz (Eds.). Headache.
Elsevier, 161–172.
Yang, A. C./Fuh, J.-L./Huang, N. E./Shia,
B.-C./Peng, C.-K./Wang, S.-J. (2011). Temporal associations between weather and
headache: analysis by empirical mode decomposition. PloS One 6 (1), e14612. https://doi.org/10.1371/journal.pone.0014612.
Zivadinov, R./Willheim, K./Sepic-Grahovac,
D./Jurjevic, A./Bucuk, M./Brnabic-Razmilic, O./Relja, G./Zorzon, M. (2003). Migraine and
tension-type headache in Croatia: a population-based survey of precipitating factors.
Cephalalgia 23 (5), 336–343. https://doi.org/10.1046/j.1468-2982.2003.00544.x.
