Dental age is an independent marker of biological age
Sonja Böker ✉
✉
University of Potsdam, Human Biology, 14469 Potsdam, Germany
Michael Hermanussen
Aschauhof, 24340 Eckernförde – Altenhof, Germany
Christiane Scheffler
University of Potsdam, Human Biology, 14469 Potsdam, Germany
DOI: https://doi.org/10.52905/hbph2021.3.24
Abstract
Background
Biological age markers are a crucial indicator of whether children are decelerated in
growth tempo. Skeletal maturation is the standard measure, yet it relies on exposing
children to x-radiation. Dental eruption is a potential, but highly debated,
radiationfree alternative.
Objectives
We assess the interrelationship between dental eruption and other maturational markers.
We hypothesize that dental age correlates with body height and skeletal age. We further
evaluate how the three different variables behave in cohorts from differing social
backgrounds.
Sample and Method
Dental, skeletal and height data from the 1970s to 1990s from
Guatemalan boys were converted into standard deviation scores, using external references
for each measurement. The boys, aged between 7 and 12, derived from different social
backgrounds (middle SES (N = 6529), low-middle SES (N = 736), low SES Ladino (N = 3653)
and low SES Maya (N = 4587).
Results
Dental age shows only a weak correlation with skeletal age (0.18) and height (0.2). The
distinction between cohorts differs according to each of the three measurements. All
cohorts differ significantly in height. In skeletal maturation, the middle SES cohort is
significantly advanced compared to all other cohorts. The periodically malnourished
cohorts of low SES Mayas and Ladinos are significantly delayed in dental maturation
compared to the well-nourished low-middle and middle class Ladino children.
Conclusion
Dental development is an independent system that is regulated by mechanisms different
to skeletal development and growth. Tooth eruption is sensitive to nutritional status,
whereas skeletal age is more sensitive to socioeconomic background.
Keywords: dental eruption, biological age, skeletal age, growth tempo, maturation, malnutrition
Conflict of Interest: There are no
conflicts of interest.
Citation: Böker, S. / Hermanussen, M. / Scheffler, C. (2021). Dental age is an independent marker of biological age. Human Biology and Public Health 3. https://doi.org/10.52905/hbph2021.3.24.
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 31-10-2021 | Accepted: 31-03-2022 | Published: 13-06-2022
Take home message for students
Dental eruption is an independent biological maturation system that is regulated by other
mechanisms than body height and skeletal age. Dental eruption is sensitive to malnutrition
and may serve as an additional tool to differentiate between malnutrition and other
reasons for impaired growth in children.
Contents
Introduction
Children are defined as stunted if their height-for-age is more than two standard
deviations below the WHO Child Growth Standards median (World Health Organization, 2015). Stunting is the impaired growth and development
that children experience from poor nutrition, repeated infection, and inadequate
psychosocial stimulation. Thus, height, weight, and body mass index are routinely used to
classify the nutritional status of children in many Low and Middle Income Countries (LMIC).
Poor nutrition does not only impair child growth, it can also delay bone maturation. For
decades, it has been known that, irrespective of socioeconomic background, bone age of
under-nourished children is delayed; the more severe the undernutrition the more delayed the
bone age (Alcázar et al., 1984). On the other hand,
stunting is not a synonym of malnutrition (Scheffler
et al., 2020). Being shorter than two standard deviations below the WHO’s standard
median is also a common feature among well-nourished and healthy children, and it is not
always evident whether short stature results from food shortage, illness, inadequate
psychosocial stimulation, or simply reflects a slower than average pace of growth and
development. One of the most common causes of short stature is the benign idiopathic delay
in developmental tempo (“late bloomer”), characterized by a substantial delay in bone age
without any signs of impaired health (Aguilar and Castano,
2022; Creo and Schwenk, 2017).
Though it is well known that different parts of the human body grow at different rates and
tempo (Prokopec, 2001), delays in developmental
tempo are usually clinically diagnosed by the delay in bone maturation as the skeletal
development is considered “the only means of assessing rates of maturational change
throughout the growing period” (Cox, 1997).
However, assessing bone maturation requires exposing children to ionizing radiation, which
poses a health risk (Meo et al., 2006).
Additionally, as mobile x-ray apparatuses are expensive and difficult to transport due to
their weight and size, they are not part of the standard equipment of anthropometric field
work.
Counting the number of teeth that have erupted through the gums is an alternate
anthropometric marker of developmental maturation. It is non-invasive and covers a
relatively long period of growth, especially considering that two sets of teeth (deciduous
and permanent) develop consecutively, three sets, if the third molars are included (Demirjian, 1986). In this study we are interested in
the permanent teeth, which traditionally are said to occur between the ages of 6 and 13
years (Logan and Kronfeld, 1933).
However, tooth eruption as a marker of biological age, and the relationship between dental
age and skeletal age, are under considerable debate. While some researchers argue that the
two maturational processes have a strong positive correlation (Al-Balbeesi et al., 2018; Demisch and
Wartmann, 1956; Liliequist and Lundberg,
1971; Sierra, 1987), Demirjian et al.
(Demirjian et al., 1985), along with various
other researchers (Beunen et al., 2006; Bielicki et al., 1984; Lewis, 1991), suggests, that tooth development is an independent system, as only
weak or even insignificant correlations were found.
In the past, radiographic dental age assessment methods have been widely employed (Demirjian et al., 1973; Kumar et al., 2013; Nolla,
1960), but little research has been done using the non-invasive approach of dental
eruption. There is a lack of dental age references, and practical methods to allow for
transforming the state of teeth eruption into any useful developmental variable (Demirjian, 1986). Our main goal for this study is to
assess the interrelationship of dental age, skeletal age and height using the number of
erupted teeth as variable for dental age. To convert these three variables into comparable
units, we used a z-transformation (standard deviation score = SDS). If the correlation
between dental age SDS and skeletal age SDS is high, it would imply that they belong to
dependent systems. We propose the following hypotheses:
| 1. | Dental age SDS (dental SDS) shows a strong positive correlation with the skeletal age
SDS (skeletal SDS). |
| 2. | Dental SDS shows a strong positive correlation with height SDS. |
| 3. | Skeletal SDS shows a strong positive correlation with height SDS. |
The correlation of dental age towards other biological age markers should not be confused
with a validation of the reliability of dental age as a biological age marker itself. To
stress this, we additionally evaluate how dental age, skeletal age and height differ
according to socioeconomic status (SES) and ethnicity in a second part. We assume that, if
dental age enables us to significantly distinguish between cohorts based on developmental
differences, it indicates a potential useful application of dental eruption as a
non-invasive biological age marker, regardless of its relationship towards other
developmental makers. We propose the following hypothesis:
| 4. | Cohorts that differ significantly in skeletal age also differ significantly in dental
age. |
For the analysis, we are using a Guatemalan dataset that is comprised of three different
social strata (middle, low to lower middle (low-middle), and low socio-economic status
(SES)). The low SES cohort divides into a Maya and a Ladino group, whereas all other strata
are Ladinos only. Both groups of the low SES cohort suffered from periods of malnutrition
(Bogin and MacVean, 1981). While there is no such
information about the low-middle SES cohort available, children of the middle SES group were
not exposed to food insecurity.
Dental information was only available for boys, females could not be analyzed.
Sample and Method
We analyzed data from the Longitudinal Study of Child and Adolescent Development by the
Universidad del Valle Guatemala (UVG). Between 1953 and 1999, various physical and cognitive
variables were measured in Guatemalan school children of different social backgrounds and
ethnicities. The two main goals of said study were firstly, understanding the processes of
growth and development over time and secondly, to provide reference data for Guatemalan
children. Back then, the regular use of x-radiation was not a concern, leading to sizable
longitudinal and cross sectional datasets on skeletal maturation of Guatemalan children and
adolescents of various social backgrounds (for more information see (Bogin and MacVean, 1983, 1984)).
We included observations of boys between the ages of 7- and 12-years with dental and/or
skeletal information obtained between the early 1970s and the late 1990s (see Table 1). Some
individuals were measured up to 4 (max 6) consecutive years, creating a mix of a
longitudinal and cross-sectional data set. Dental information is given as the sum of
permanent teeth which had at least perforated the gum with any part of the crown. This was
examined by a clinical dentist. Skeletal information is given as bone age (Greulich and Pyle, 1959), using x-ray pictures of the
left hand and wrist.
Table 1 Guatemalan boys with information on permanent teeth (dental data) and/or a
skeletal age (skeletal data). Cohorts are separated by socioeconomic status (SES) and
ethnicity.
| SES |
Ethnicity |
Dental data N |
Skeletal data N |
Exposed to Malnutrition |
School Fee |
| Middle |
Ladino |
4157 |
2540 |
No |
Yes |
| Low-Middle |
Ladino |
716 |
90 |
Unknown |
Yes |
| Low |
Ladino |
2285 |
1520 |
Yes |
No |
| Low |
Maya |
4302 |
1219 |
Yes |
No |
For each child, we determined a “dental age”, based on a Cuban reference that provided mean
age and standard deviations for teeth eruption and was considered appropriate for Guatemalan
children (San Miguel Pentón et al., 2011), and the
“skeletal age” according to Greulich & Pyle. Dental age and skeletal age were
transformed into z-scores,
where x = individual age, µ = mean age of the reference when the respective individual
state of dental and skeletal maturity was reached, σ = standard deviation of the reference).
Height was transformed into z-scores based upon WHO-references. For the final analysis, we
only considered children with maximum 27 teeth, since dental maturity scores for assessing
the process of maturation are only meaningful if dentition has not been completed. Z-scores
are referred to as SDS (standard deviation scores).
Statistics
The open source program RStudio (R version 4.0.2, R core team, 2020) was used for all
analyzes.
To assess the interrelationship between height-, bone age- and dental age SDS, a
correlation matrix (spearman) and linear models were implemented.
To assess differences between cohorts, requirements for parametric tests were checked as
follows: normal distribution was tested using the Shapiro-Wilk-Test, followed by a visual
verification with QQ-plots. The Levene-Test was used to test for homogeneity of variance.
Neither skeletal-, dental-, nor height SDS met the latter criteria. Dental z-scores did
not follow a normal distribution. We used Kruskal-Wallis-Test for analyzing differences
between groups. Pairwise comparison was done with the Dunn-Test, using the multiple
comparison adjustment according to the Bonferroni method.
Results
Part 1: Correlation Between Height-, Skeletal- and Dental SDS
With r = 0.18, there is a significant (p = 0.00) but weak correlation between dental age
SDS (dental SDS) and skeletal age SDS (skeletal SDS). The correlation between dental SDS
and height SDS is also weak (r = 0.20, p = 0.00). Height and skeletal maturity show a
moderate to high positive correlation (r = 0.58, p = 0.00).
The correlations per cohort are shown in Table 2. The low SES Ladinos show the weakest
correlation between dental and height SDS (r = 0.16) and dental and skeletal SDS (r =
0.08), the latter being not significant. The low-middle SES Ladinos show the strongest
correlation between dental and height SDS (r = 0.32) and dental and skeletal SDS (r =
0.29). The correlation between height and skeletal SDS ranges between r = 0.56 (middle SES
Ladinos) and r = 0.66 (low SES Ladinos). All correlations are significant, apart from the
one exception mentioned above.
Table 2 Correlation matrix between height-, skeletal- and dental SDS per cohort (midLad
= middle SES Ladinos, lowmidLad = low-middle SES Ladinos, lowLad = low SES
Ladinos, lowMaya = low SES Maya).
| Cohort |
|
Height SDS |
Skeletal SDS |
Dental SDS |
| midLad |
Height SDS |
1 |
|
|
| Skeletal SDS |
0,56 |
1 |
|
| Dental SDS |
0,25 |
0,16 |
1 |
| lowmidLad |
Height SDS |
1 |
|
|
| Skeletal SDS |
0,61 |
1 |
|
| Dental SDS |
0,32 |
0,29 |
1 |
| lowLad |
Height SDS |
1 |
|
|
| Skeletal SDS |
0,66 |
1 |
|
| Dental SDS |
0,16 |
0,08 |
1 |
| lowMaya |
Height SDS |
1 |
|
|
| Skeletal SDS |
0,57 |
1 |
|
| Dental SDS |
0,29 |
0,23 |
1 |
Figure 1 illustrates the relation between skeletal
and height SDS, which follows a clear positive linear relationship. Yellow dots indicate
dental SDS equal or above -2, purple dots indicate dental SDS below -2. The purple dots
are almost equally scattered and highlight the lack of association between dental SDS and
height SDS or skeletal SDS.
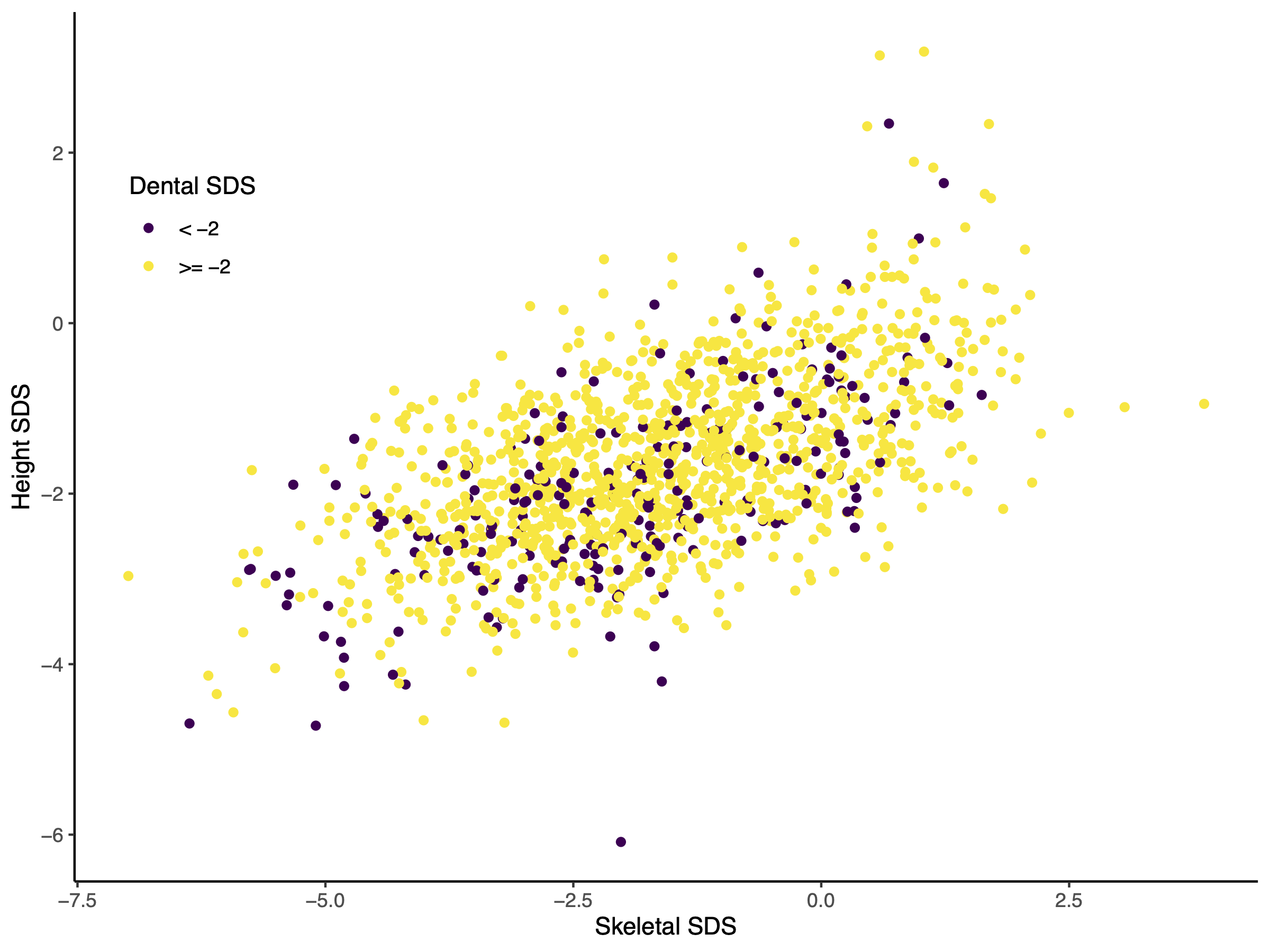
Figure 1 Scatter Plot of skeletal SDS against height SDS of all Guatemalan boys. Red dots
indicate individuals that are delayed in dental eruption (dental SDS below -2).
Part 2: The effect of socioeconomic status (SES) and ethnicity (Ladino, Maya) on
dental age, skeletal age and height
Social strata and ethnicities (Table 1) differ in height SDS, skeletal age SDS and dental
ages SDS (Figure 2). Height SDS differs between all
schools (pairwise comparison, p < .001, Table 4). Well-nourished middle SES Ladino children were significantly advanced in
height, in skeletal age, and in dental age. However, Figure 2 illustrates that the magnitude of this advancement differed between
the three variables. Whereas the middle SES children were taller and appeared “older” in
skeletal age, the advancement in dental age was small (see also Table 3 for the mean and standard deviation per measurement
and cohort). Yet, the leptokurtic distribution of dental SDS reached significance and
indicates that the periodically malnourished cohorts of low SES Mayas and Ladino children
advanced in dental maturation at slower pace than the well-nourished low-middle and middle
class Ladino children.
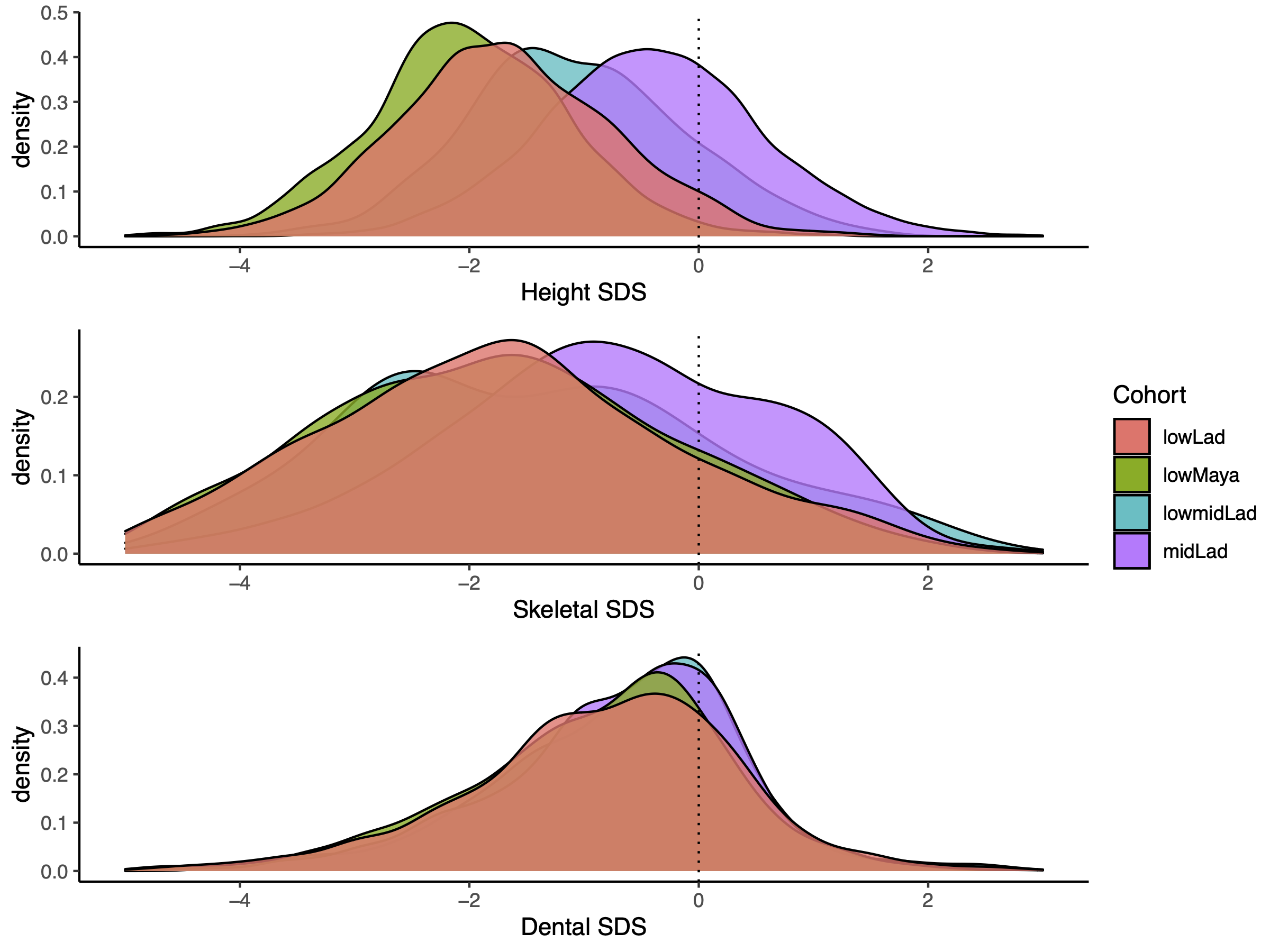
Figure 2 Distribution of SDS for each measurement (dental-, height-, skeletal) per cohort
(midLad = middle SES Ladinos, lowmidLad = low-middle SES Ladinos, lowLad = low SES
Ladinos, lowMaya = low SES Maya), Within all measurements, there are significant
differences between cohorts. Kruskal Wallis Test results: Height SDS: H(3) = 6050.6, p
< .001, skeletal SDS: H(3) = 480.02, p < .001, dental SDS: H(3) = 79.586, p <
.001.
Table 3 Mean and standard deviation for height, skeletal and dental SDS per cohort
(midLad = middle SES Ladinos, lowmidLad = low-middle SES Ladinos, lowLad = low SES
Ladinos, lowMaya = low SES Maya).
| SDS |
Cohort |
mean |
sd |
Kurtosis |
skewness |
N |
| Height |
midLad |
-0,41 |
0,97 |
0,36 |
0,14 |
6529 |
| lowmidLad |
-1,06 |
0,96 |
1,43 |
0,42 |
736 |
| lowLad |
-1,68 |
0,96 |
0,31 |
0,13 |
3653 |
| lowMaya |
-2,01 |
0,89 |
1,24 |
0,15 |
4587 |
| Skeletal age |
midLad |
-0,48 |
1,32 |
0,22 |
-0,09 |
2540 |
| lowmidLad |
-0,82 |
1,7 |
3,38 |
1,22 |
90 |
| lowLad |
-1,31 |
1,45 |
0,89 |
0,28 |
1520 |
| lowMaya |
-1,34 |
1,42 |
0,17 |
0,11 |
1219 |
| Dental age |
midLad |
-0,68 |
1,16 |
4,66 |
0,16 |
4175 |
| lowmidLad |
-0,64 |
1,22 |
7,16 |
0,68 |
716 |
| lowLad |
-0,85 |
1,27 |
2,40 |
-0,40 |
2285 |
| lowMaya |
-0,89 |
1,21 |
2,49 |
-0,51 |
4302 |
Table 4 Summary table of adjusted p-values from the pairwise comparison between cohorts
for height-, skeletal-, and dental SDS (midLad = middle SES Ladinos, lowmidLad =
low-middle SES Ladinos, lowLad = low SES Ladinos, lowMaya = low SES Maya).
| School Pairs |
Height SDS |
|
Skeletal SDS |
|
Dental SDS |
|
| midLad x lowLad |
p < 0,001 |
*** |
p < 0,001 |
*** |
p < 0,001 |
*** |
| midLad x lowmidLad |
p < 0,001 |
*** |
p = 0,01 |
* |
p = 1,00 |
|
| lowLad x lowmidLad |
p < 0,001 |
*** |
p = 0,08 |
|
p = 0,002 |
** |
| midLad x lowMaya |
p < 0,001 |
*** |
p < 0,001 |
*** |
p < 0,001 |
*** |
| lowLad x lowMaya |
p < 0,001 |
*** |
p = 1,00 |
|
p = 1,00 |
|
| lowmidLad x
lowMaya |
p < 0,001 |
*** |
p = 0,06 |
|
p < 0,001 |
*** |
Discussion
Low SES Mayas are short. Their shortness is associated with very poor social conditions
(Bogin and MacVean, 1984) and closely corresponds
with a delay in skeletal age SDS. However, the correlations between dental maturation and
height and dental maturation and skeletal age are low and explain less than 10 % of the
variance (3 % and 7 % respectively).
We reject both hypothesis 1, that “dental SDS shows a strong positive correlation with
skeletal SDS”, and hypothesis 2, that “dental SDS shows a strong positive correlation with
height SDS”.
The results suggest that the progress in dental age is independent of skeletal age and is
instead regulated by different mechanisms. While this is supported by findings in other
human populations (Bielicki et al., 1984; Demirjian et al., 1985), these reports are not
univocal. Several studies appear to show a dependency between bone age and tooth
development. This is difficult to explain and may be due to the lack of standardized methods
for analyzing age-dependent variables (Lewis 1991).
Growth depends on bone formation (Beunen et al.,
2006; Demirjian et al., 1985). Growth in
height largely depends on the formation of longitudinal bones, and thus, on epiphyseal
growth that is similar in femur and tibia and in the phalanges. Growth of the mid-face and
the teeth differs and appears less sensitive than skeletal growth to environmental
influences, such as socioeconomic strata (SES) and ethnicities.
Yet, we find differences between those, had to pay a school fee (midLad, lowmidLad) and
those who did not (lowLad, LowMaya), the former showing a small but significant advancement.
Previous studies on the same Guatemalan dataset state that low SES Ladino children and
especially low SES Maya children suffered from periods of malnutrition (Bogin and MacVean, 1981). While we did not reassess
nutritional status in the presented study, we suspect that malnutrition might be the driver
for the delay in dental eruption in those two cohorts. This would align with previous
findings that identified nutritional status as the main effector of dental development,
while other environmental influences, such as social status, show no significant impact
(Alhamda, 2012; Demirjian, 1986; Psoter et al., 2008).
There is one major misconception in analyzing the reliability of biological age markers,
that we also failed to realize. Namely the notion that developmental markers must produce
results similar to skeletal age in order to be considered a good indicator for biological
age. We expected such a signal while formulating hypothesis 4: “Cohorts that differ
significantly in skeletal age, also differ significantly in dental age”, which we had to
reject.
Different organ systems develop at different rates (Beunen
et al., 2006). There is not one single “biological age” that can be identified by
x-rays of the hand and wrist. “Skeletal age” identified in long bones is not congruent with
the state of maturity in dentition. Instead, the present study suggests that even within the
skeletal apparatus more than one “biological age” exists. We question that bone age can
serve as a “gold standard” of biological age.
Conclusion
Dental eruption is an independent biological maturation system that is regulated by other
mechanisms than skeletal age and height. Dental eruption seems to be is sensitive to
malnutrition and may serve as an additional tool to differentiate between malnutrition and
other reasons for impaired growth in children, whereas skeletal age is more sensitive to
socioeconomic background. In future studies the relationship between nutritional status and
dental eruption should be further analyzed.
References
Aguilar, D./Castano, G. (2022). Constitutional
Growth Delay, in: StatPearls. StatPearls Publishing, Treasure Island
(FL).
Al-Balbeesi, H.O./Al-Nahas, N.W./Baidas, L.F./Bin
Huraib, S.M./Alhaidari, R./Alwadai, G. (2018). Correlation between skeletal maturation
and developmental stages of canines and third molars among Saudi subjects. The Saudi
dental journal 30, 74–84. https://doi.org/10.1016/j.sdentj.2017.11.003
Alcázar, M.L./Alvear, J./Muzzo, S. (1984).
Influence of nutrition on the bone development of children. Archivos Latinoamericanos de
Nutricion 298–307.
Alhamda, S. (2012). Relationship between
nutritional status and eruption of first permanent mandibular molar teeth among the
school children in Indonesia. South East Asia Journal Of Public Health 85–86. https://doi.org/10.3329/seajph.v2i2.15962
Beunen, G.P./Rogol, A.D./Malina, R.M. (2006).
Indicators of Biological Maturation and Secular Changes in Biological Maturation. Food
and Nutrition Bulletin 244–256. https://doi.org/10.1177/15648265060274S508.
Bielicki, T./Koniarek, J./Malina, R.M. (1984).
Interrelationships among certain measures of growth and maturation rate in boys during
adolescence. Annals of human biology 11, 201–210. https://doi.org/10.1080/03014468400007071
Bogin, B./MacVean, R.B. (1984). Growth status of
non- agrarian, semi-urban living Indians in Guatemala. Human Biology
527–538.
Bogin, B./MacVean, R.B. (1983). The Relationship
of Socioeconomic Status and Sex to Body Size, Skeletal Maturation, and Cognitive Status
of Guatemala City Schoolchildren. Child Development 115–128.
Bogin, B./MacVean, R.B. (1981). Nutritional and
Biological Determinants of Body Fat Patterning in Urban Guatemalan Children. Human
Biology 259–268.
Cox, L. (1997). The biology of bone maturation and
ageing. Acta Paediatrica 86, 107–108. https://doi.org/10.1111/j.1651-2227.1997.tb18386.x
Creo, L.A./Schwenk, W.F. (2017). Bone age: a handy
tool for pediatric providers. Pediatrics. https://doi.org/10.1542/peds.2017-1486
Demirjian, A. (1986). Dention, in: Falkner, F.,
Tanner, J.M. (Eds.), Postnatal Growth Neurobiology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4899-0522-2‗ 12
Demirjian, A./Buschang, P.H./Tanguay,
R./Patterson, D.K. (1985). Interrelationships among measures of somatic, skeletal,
dental, and sexual maturity. American Journal of Orthodontics 433–438. https://doi.org/10.1016/0002-9416(85)90070-3
Demirjian, A./Goldstein, H./Tanner, J.M. (1973). A
New System of Dental Age Assessment. Human Biology 211–227.
Demisch, A./Wartmann, P. (1956). Calcification of
the Mandibular Third Molar and Its Relation to Skeletal and Chronological Age in
Children. Child Development. https://doi.org/10.1111/j.1467-8624.1956.tb04824.x
Greulich, W./Pyle, S. (1959). Radiographic atlas
of skeletal development of the hand and wrist. Stanford university
press.
Kumar, V./Venkataraghavan, K./Krishnan, R./Patil,
K./Munoli, K./Karthik, S. (2013). The relationship between dental age, bone age and
chronological age in underweight children. Journal of pharmacy & bioallied sciences
73–79. https://doi.org/10.4103/0975-7406.113301
Lewis, A.B. (1991). Comparisons between dental and
skeletal ages. The Angle Orthodontist 87–92. https://doi.org/10.1043/0003-3219(1991)061\textless0087:CBDASA\textgreater2.0.CO;2
Liliequist, B./Lundberg, M. (1971). Skeletal and
tooth development: A methodological investigation. Acta Radiologica
97–112.
Logan, W.H.G./Kronfeld, R. (1933). Development of
the Human Jaws and Surrounding Structures from Birth to the Age of Fifteen Years**From
the Research Department of the Chicago College of Dental Surgery, Dental Department of
Loyola University.Read at the Third General Meeting of the Seventy-Fourth Annual Session
of the American Dental Association, Buffalo, N. Y., Sept. 14, 1932. The Journal of the
American Dental Association (1922) 20, 379–428. https://doi.org/10.14219/jada.archive.1933.0080
Meo, S.A./Al Drees, A.M./Zadi, S.Z./Damgh,
S.A./Al-Tuwaijri, A.S. (2006). Hazard of X-Ray Radiation on the Quantitative and
Phagocitc Function of Polymorphonuclear Neutrophils in X-Ray Technicians. Journal of
Occupational Health 88–92. https://doi.org/10.1539/joh.48.88
Nolla, C.M. (1960). The Development of the
Permanent Teeth. Journal of Dentistry for Children 254–266.
Prokopec, M. (2001). Differential rate of growth
of the human body parts., in: Dasgupta, P./Hauspie, R. (Eds.), Perspectives in Human
Growth, Development and Maturation. Springer, Dordrecht. https://doi.org/10.1007/978-94-015-9801-9_24
Psoter, W./Gebrian, B./Prophete, S./Reid, B./Katz,
R. (2008). Effect of early childhood malnutrition on tooth eruption in Haitian
adolescents. Community dentistry and oral epidemiology 36, 179–189. https://doi.org/10.1111/j.1600-0528.2007.00386.x
San Miguel Pentón, A./Veliz Concepción,
O.L./Escudero Alemán, R.Z./Calcines Ferrer, M.E./Ortega Romero, L. (2011). Cronología de
emergencia de la dentición permanente en niños del municipio de Santa Clara: Parte I:
Permanent dentition emergence chronology in children from Santa Clara municipality: Part
I. Revista Cubana de Estomatol 208–218.
Scheffler, C./Hermanussen, M./Bogin, B./Liana,
D.S./Taolin, F./Cempaka, P.M.V.P./Irawan, M./Ibbibah, L.F./Mappapa, N.K./Payong,
M.K.E./Homalessy, A.V./Takalapeta, A./Apriyanti, S./Manoeroe, M.G./Dupe, F.R./Ratri,
R.R.K./Touw, S.Y./K, P.V./Murtani, B.J./Nunuhitu, R./Puspitasari, R./Riandra,
I.K./Liwan, A.S./Amandari, P./Permatasari, A.A.I./Julia, M./Batubara, J./Pulungan, A.
(2020). Stunting is not a synonym of malnutrition. European journal of clinical
nutrition 74, 377–386. https://doi.org/10.1038/s41430-019-0439-4
Sierra, A.M. (1987). Assessment of dental and
skeltal maturaty: A new approach. The Angle Orthodontist 194–208.
World Health Organization (2015). Stunting in a
nutshell, [WWW Document]. URL https://www.who.int/news/item/19-11-2015-stunting-in-a-nutshell

