Greater central adiposity resulting from increased market integration is
associated with elevated C-reactive protein levels in older women from the Republic of
Vanuatu
Hayley Mann ✉
✉
Department of Anthropology, Binghamton University, Binghamton, NY
Laboratory of Evolutionary Anthropology and Health, Binghamton University,
Binghamton, NY
Department of Chronic Disease Epidemiology, Yale School of Public Health, New
Haven, CT
Department of Anthropology, New Mexico State University, Las Cruces NM
Department of Anthropology, Binghamton University, Binghamton, NY
Laboratory of Evolutionary Anthropology and Health, Binghamton University,
Binghamton, NY
Laboratory of Biomedical Science, The Feinstein Institute for Medical
Research, Manhasset, NY
Hofstra North Shore-LIJ School of Medicine at Hofstra University, Hempstead,
NY
Départment des sciences de l’activité physique, Université du Quebec à
Montréal, Montréal QC, Canada
Department of Parasitology, Graduate School of Medicine, Osaka City
University, Osaka, Japan
Ministry of Health, Port Vila, Vanuatu
Ministry of Health, Port Vila, Vanuatu
Department of Parasitology, Graduate School of Medicine, Osaka City
University, Osaka, Japan
Island Malaria Group, Department of Microbiology, Tumor and Cell Biology
(MTC), Karolinska Institutet, Stockholm, Sweden
Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan
Department of Anthropology, Temple University, Philadelphia, PA
Department of Anthropology, Binghamton University, Binghamton, NY
Laboratory of Biomedical Anthropology and Neurosciences, Binghamton
University, Binghamton, NY
Department of Biological Sciences, Binghamton University, Binghamton,
NY
Department of Anthropology, Binghamton University, Binghamton, NY
Laboratory of Evolutionary Anthropology and Health, Binghamton University,
Binghamton, NY
Department of Biological Sciences, Binghamton University, Binghamton,
NY
DOI: https://doi.org/10.52905/hbph.v2.20
Abstract
Objective
We characterized the relationship between circulating C-reactive protein (CRP) levels
and nine anthropometric measures of body composition to identify the best anthropometric
predictors of CRP in Ni-Vanuatu women.
Sample and Methods
Anthropometric data and blood spot samples were collected from sixty-four Ni-Vanuatu
female participants (age 35-78 years) on five islands with varying degrees
of market integration, cultural change, and obesity. CRP concentration was determined
with a high-sensitivity ELISA (hsCRP) assay and then compared to nine different
anthropometric measurements.
Results
BMI was significantly correlated with CRP (p=0.047, r2=0.249). Among the
eight additional anthropometrics, BIA (p=0.040, r2=0.257),
waist-circumference (p=0.009, r2=0.325) and suprailiac skinfold (p=0.003,
r2=0.373) were better predictors of CRP than BMI. Moreover, our stepwise
selection model indicated that the suprailiac skinfold explained ~14% of CRP level
variance.
Conclusions
The BMI-CRP correlation coefficient for Ni-Vanuatu women falls within the range of
previously reported values for East Asian populations with whom they share genetic
ancestry. Furthermore, the best anthropometric predictors of CRP levels were waist
circumference and suprailiac skinfold thickness. These measures capture central
adiposity and are more closely associated with elevated CRP level and cardiovascular
disease risk than fat distributed elsewhere on the body. Ni-Vanuatu in urban
settings with high market integration are at greater risk for
obesity, which is associated with elevated CRP levels. However, because nearly all
Ni-Vanuatu still retain horticultural knowledge and land ownership, consumption of
processed, imported foods is largely determined by degree of market integration and
personal choice. Therefore, health interventions focusing on sustainable traditional
food practices are feasible.
Keywords: Body Mass Index, central adiposity, Pacific Islands, Melanesians, market integration
Conflict of Interest: There are no
conflicts of interest.
Citation: Mann, H. et al. (2021). Greater central adiposity resulting from increased market integration is
associated with elevated C-reactive protein levels in older women from the Republic of
Vanuatu, Human Biology and Public Health 2. https://doi.org/10.52905/hbph.v2.20.
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 23-06-2021 | Accepted: 17-08-2021 | Published: 22-12-2021
Take home message for students
High obesity rates in the Pacific Islands are correlated with processed foods which are
available via increasing market integration. CRP level is most correlated with central
adiposity, and waist circumference is most conveniently measured in the field. Since
nearly everyone owns gardens, consumption of processed foods is a personal choice in
Vanuatu.
Contents
Introduction
Since the 1980s, global obesity prevalence has doubled and is now considered a universal
human health problem (WHO Expert Consultation
2004). Moreover, 55% of this global rise in mean body mass index (BMI) occurred in
rural areas. For low- and middle-income countries, data also suggests that rural mean BMI is
rising at similar rates to urban settings, especially for women (NCD Risk Factor Collaboration 2019). The marketing of inexpensive,
processed food has resulted in the overconsumption of nutrient poor dietary items, while
lifestyle changes associated with modernization has led to a decrease in physical activity
(Popkin et al. 2012; Swinburn et al. 2011). Currently, the Pacific Islands have some of the
world’s highest rates of obesity (Gill 2006). Many
Pacific Island nations are lower income with poor infrastructure. Some islands also have a
double burden of non-communicable diseases (NCDs) and infectious diseases, which makes
public health interventions more challenging. Furthermore, Pacific Islanders are
significantly underrepresented in health literature and are a medically underserved
population with unequal access to the healthcare (Ghosh 2003).
Long periods of colonization, de-population from introduced diseases, resource exploitation
and more recently, global market integration have resulted in a series of sociocultural
changes that continue to negatively impact indigenous health throughout the Pacific. In
Melanesia, European contact began in the 1500s and first contacts by explorers,
missionaries, whalers, sandalwooders, and miners continued through the 20th century.
Indigenous people suffered various devastations including the loss of traditional lands and
culture, and the introduction of new diseases (Allen
1968; MacClancy 1981). In the second half
of the nineteenth century, exploitation of Melanesians for plantation/cash crop labor
intensified while epidemics and population loss continued (MacClancy 1981). Allied and Japanese forces occupied much of Melanesia during
World War II, significantly increasing infrastructure and the availability of imported
processed foods in some regions. By the end of WWII, post-contact dietary items such as
cooking oil, refined sugar, tinned meats, beef, dairy products, and bread had become food
staples on some of the islands (Dancause et al.
2011; 2013). Beginning with Papua New Guinea
in 1975, many pacific island nations gained their independence. On larger islands, urban
administrative and commercial centers emerged characterized by greater infectious disease
control, tourism, and more convenient food choices, but poor nutrition and exercise
resulting in higher obesity (Dancause et al. 2011).
Obesity therefore became a public health concern in Melanesia after WWII and has continued
to increase over time.
Currently, the ten countries with the highest rates of adult obesity are all located in
Micronesia and Polynesia, with Nauru (61%) and the Cook Islands (55.9%) being the top two
(Central Intelligence Agency 2021). Between
1980–2008, mean BMI in the Cook Islands and Nauru increased at four times the global rate
(McLennan and Ulijaszek 2015). The countries
comprising Melanesia have less obesity; especially in endemic malarious regions. For
example, Vanuatu, the Solomon Islands and Papua New Guinea have obesity rates of 25.2%,
22.5% and 21.3%, respectively (Central Intelligence Agency
2021). Although the low- and middle-income countries of the Western Pacific Region
have lower obesity rates, non-communicable diseases (NCDs) such as cardiovascular diseases
(CVDs), diabetes and cancer still account for 50% of premature deaths in individuals under
70 years of age (Shin and Varghese 2014). With
improved infectious disease control measures on some of the islands (Kaneko et al. 2000), increases in tourism (Dancause et al. 2011) and worsening climate change (Swinburn et al. 2011), the obesity rate in Melanesia
will likely approach those of Micronesia and Polynesia.
To help address NCD-related public health concerns, research in the Western Pacific region
has focused on analyzing anthropometric measures within a context of changing behaviors and
physical activities (Dancause et al. 2011; Olszowy et al. 2015). Individual obesity has been
estimated in a variety of ways for different studies. BMI and waist circumference thresholds
are commonly used to assess individual CVD risk (DeLoach
et al. 2014; Ding et al. 2015; Schafer et al. 2011). However, these measurements
cannot distinguish fat mass from fat-free mass, so the corresponding estimated CVD risk
category may not be accurate for some individuals, particularly athletes (Dancause et al. 2010; Klein et al. 2007; Neovius et al. 2006).
In comparison to North American and European populations, Asian populations have a greater
predisposition for developing abdominal obesity and a higher risk for developing CVDs at
lower BMIs (Forouhi et al. 2001; WHO Expert Consultation 2004). This observation led to
the development of different BMI cutoff points in various Asian populations; specifically,
the BMI obesity cutoff value for health intervention was lowered (Popkin 2004; WHO Expert Consultation
2004). Likely reflecting their shared genetic ancestry with East Asians, Pacific
Islanders also possess greater abdominal obesity (Dancause
et al. 2011). It has therefore been suggested that the World Health Organization
(WHO) action guidelines for Asian populations should also be applied to Pacific Islanders in
Western Melanesia (Dancause et al. 2010). For a more
precise measure of body composition, bioelectrical impedance analysis (BIA) has also been
used to determine body fat percentage. Limitations of BIA equipment include reliance on
technology, variable body electrode placement, and also different body fat percentage
prediction models that are dependent upon sex, body type, population-specific equations, and
other assumptions (Dancause et al. 2010; Neovius et al. 2006; Sergi et al. 2017). Skinfold thickness anthropometrics provide a more direct
measure of fat tissue, but there is less population data associated with skinfold measures
and the usage of calipers may be uncomfortable for some participants. Ultimately, measuring
individual BMI and waist circumference may be more feasible in remote clinical and field
site settings.
Chronic low-grade inflammation associated with obesity is also known to play a major role
in the development of cardiovascular diseases (Brooks
et al. 2010; Khoo et al. 2011; Pearson et al. 2003). C-reactive protein (CRP) is an
inflammatory response biomarker and assessing CRP levels improves the accuracy of predicting
cardiovascular disease-related outcomes in patients (Brooks
et al. 2010; Danesh et al. 2000; Pearson et al. 2003). CRP testing is widely utilized by
human biologists and medical researchers in their studies to better characterize the
relationships among obesity, elevated CRP level and CVD outcomes across different
populations (Choi et al. 2012; Khoo et al. 2011; McDade et al.
2009; Sung et al. 2014). The American
Heart Association (AHA) has provided recommendations for using high sensitivity CRP (hsCRP)
testing to help identify CVD risk when included with measures of adiposity. Currently, the
AHA’s three CRP categories for CVD risk are low (<1.0 mg/L), intermediate (1.0–2.9 mg/L),
and high (>3.0 mg/L). Elevated CRP level (i.e., above 1.0 mg/L) is an indication of
low-grade inflammation associated with obesity and risk for developing CVDs (Pearson et al. 2003). However, the cross-population
clinical utility of currently recommended AHA CRP category cutoff values for CVD risk has
been questioned (Sung et al. 2014). When
controlling for BMI and other CVD-related risk factors (e.g., smoking), average CRP levels
for East and Southeast Asian populations are lower than American, European, and South Asian
populations (Choi et al. 2012; Khoo et al. 2011; McDade et al.
2009; Sung et al. 2014). McDade et al. (2009) reported that American women are 3.9 times more likely to have a high-risk
CRP concentration of >3.0 mg/L than Filipino women with the same BMI. A lower CRP level
average in East Asians, however, does not necessarily mean the risk for developing a CVD is
reduced. For instance, Sung et al. (2014) showed that a Korean population with
predominantly low CRP levels (<1.0 mg/L) had an elevated risk for CVD-related events with
only minor increases in CRP level. It has also been observed that populations differ in
regard to how strongly BMI correlates with CRP level. In particular, East Asian populations
tend to have a weaker association between BMI and CRP levels than North American or European
populations (Choi et al. 2012; Doumatey et al. 2010; Jeemon et al.
2011; Lim et al. 2006; Saito et al. 2003). These studies show that ancestry
can modify the relationship between CRP and CVD risk, which raises the possibility that the
standardized CRP-associated CVD risk categories may be underestimating the number of
individuals who have an elevated risk for CVD in certain populations (Sung et al. 2014).
This study includes Ni-Vanuatu women residing on five different islands of The Republic of
Vanuatu. Amongst islands of Vanuatu, there is a gradient of infectious disease control and
market access to imported and processed food items. Along with low genetic diversity among
Ni-Vanuatu, the characteristic variation of rural and more urbanized islands can be studied
as a natural experimental model (Dancause et al.
2011). Although processed foods are currently available throughout Vanuatu, nearly
all Ni-Vanuatu still retain traditional land (due to historical, disease-induced
depopulation) and gardens resulting in minimal food insecurity, which is an uncommon feature
for a lower-income county. Research in Vanuatu has provided valuable insight into human
health transitions (Dancause et al. 2011; Olszowy et al. 2015). As yet, efforts to assess the
relationship between body composition and CRP level in Melanesian populations have been
limited. The current study is our first attempt to add protein biomarker analyses to our
ongoing longitudinal examination of the epidemiological transition underway in Vanuatu that
we have monitored since 2007 (Dancause et al. 2010).
We examined the relationship between nine anthropometric measures and CRP levels in blood
spots collected from older Ni-Vanuatu women age 35–72 from different islands with different
degrees of market integration. Previous analyses focused on surveys of diet and behavior, as
well as anthropometric measurements of body composition, blood pressure, and other chronic
disease correlates. These demonstrated that older women displayed the greatest variation in
health metrics. For this initial analysis, older and mainly postmenopausal Ni-Vanuatu women
participants were chosen with a wide range of BMIs because they were expected to result in a
broader range of CRP levels than less variable younger women (Sites et al. 2002; Visser et al.
1999) or men (Choi et al. 2012). Because
BMI is widely used in clinical research and CVD risk assessment across different populations
(Peters et al. 2018), CRP levels for Ni-Vanuatu
women were first analyzed within the recommended standard WHO BMI categories (World Health Organization
2000a). Next, we evaluated which anthropometric measurements of body composition were the best
predictors of CRP levels. In comparison to measures of peripheral and upper body adiposity
or whole-body anthropometric measures, we hypothesized that more direct measurements of
central adiposity would better predict CRP levels in Ni-Vanuatu women and thus, be better
measures of CVD risk than BMI.
Samples and Methods
Study population
Current patterns of genetic, linguistic, geographic affinities, and analyses of ancient
DNA indicate that Ni-Vanuatu are admixed descendants of two distinct populations; initial
Holocene (~3 kya) settlers from Island Southeast Asia and extensive post-settlement gene
flow from neighboring Pleistocene settlers of Near Oceania (Kayser 2010; Lipson et al.
2018; Lum and Cann 1998; Skoglund et al. 2016). Currently, some Ni-Vanuatu
populations possess genetic markers associated with both Melanesia and Polynesia (Vilar 2010), reflecting a 1000-year history of
settlement and ongoing gene flow between the regions. Vanuatu is also currently
experiencing economic development and cultural change at varying rates across the
archipelago (Olszowy et al. 2015). Of the five
islands surveyed, the most economically developed island is Efate which includes the urban
and malaria-free capital, Port Vila. Nguna is located just off the north shore of Efate
with easy access to Port Vila infrastructure and market resources. Aneityum is
geographically rural but has been experiencing increased tourism and development due to
the elimination of malaria in 1991 (Kaneko et al.
2000). The islands Futuna and Ambae are also rural, and villages are comprised of
extended families practicing subsistence horticulture. At the time of sample collection in
2011, both Ambae and Nguna were burdened by malaria. Although European contact with
Vanuatu first occurred during the early 1600s, major dietary transitions did not occur
until the introduction of French and British food items by colonialists in the late 1800s
(e.g., cooking oil, refined sugar, beef, dairy products, and bread) (Dancause et al. 2011; 2013).
Currently, some processed foods can be purchased throughout Vanuatu; however, most
Ni-Vanuatu still possess traditional horticultural knowledge and own land that is suitable
for sustainable, continuous food production.
Anthropometric measures of body fat
In June and July of 2011, nine different anthropometric measures were collected from
Ni-Vanuatu women participants (n=242), who resided on one of five islands of Vanuatu
(Ambae, Futuna, Aneityum, Nguna, and Efate). Everyone who wanted to participate in the
study were invited to do so. Skinfold measurements were taken to the nearest 1 mm in
triplicate using Lange skinfold calipers (Cambridge, MA) and circumferences were taken in
triplicate using non-elastic anthropometric tapes to the nearest 0.1 cm; the mean of all
measurements at each site were used in analyses. All measurements were completed according
to standard accepted guidelines (Lohman et al.
1988). Peripheral fat measurements included: upper and lower arm circumferences,
mid-lower arm skinfold (maximum circumference of the lower arm), and triceps skinfold.
Subscapular skinfold thickness was measured as an estimate of upper body fat. Central
adiposity was estimated from suprailiac skinfold thickness and waist circumference
(approximately 2 cm above the navel). Participant weight and height were measured for BMI
(weight [kg]/height squared [m2]). Percent body fat was also determined via
BIA, where electrode placement was at the feet resulting in an electrical current passing
through the abdomen (Tanita Body Composition Analyzer digital scale #TBF-521 (Arlington
Heights, IL). BIA was therefore predominantly a measure of lower body fat.
Table 1 Description of anthropometricsa and Pearson correlation coefficients with
CRP
|
Mid-lower arm skinfold |
Subscapular skinfold |
Triceps skinfold |
Lower arm circ. |
Upper arm circ. |
BMI |
BIA |
Waist circ. |
Suprailiac skinfold |
| Body locationb |
peripheral |
upper body |
peripheral |
peripheral |
peripheral |
total body |
lower body |
central |
central |
| r2
|
0.131 |
0.174 |
0.190 |
0.209 |
0.223 |
0.249 |
0.257 |
0.325 |
0.373 |
| p-value |
0.302 |
0.176 |
0.133 |
0.098 |
0.077 |
0.047* |
0.040* |
0.009** |
0.003** |
| n |
64 |
62 |
64 |
64 |
64 |
64 |
64 |
63 |
62 |
Blood spot filter collection and study subset selection
In addition to anthropometrics, filter paper blood spot samples were collected from the
same Ni-Vanuatu women participants. Blood spots were dried, placed in zip lock bags for
transport, and then were exposed to ambient tropical temperatures for up to a month before
being stored at -80ºC. For this study, sixty-four blood spot samples from female
participants age 35–76 (Suppl. Table S1) were
selected based on BMI variation (16.2-47.7 kg/m2). To evaluate if the sample
subset (n=64) was representative of the larger dataset from 2011 (n=242), three
characteristics of each dataset were compared to test the null hypothesis of no
difference. First, the means of the BMIs were compared using a two-sample t-test which
resulted in t=0.367, p=0.715. Second, the variances of the BMIs were compared using a
chi-square test which resulted in χ2= 74.95, p= 0.250. Individual BMIs were
then classified into one of four internationally utilized categories (WHO, 2000a):
underweight, average, overweight, and obese (Suppl. Table S2). These classification distributions were compared by a chi-square test
which resulted in χ2= 2.1, p= 0.549. None of the three hypotheses can be
rejected, indicating the subset analyzed for CRP is a minimally biased representation of
the larger population dataset.
CRP analysis
A 3.2mm disc from each blood spot filter paper (n=64) was taken and eluted overnight at
4oC in 250 μl of PBS Tween-20 (1 ml/L) solution followed by 300 rpm shaking
at room temperature for 60 minutes the next day (McDade et al., 2004). The eluate was
diluted to 1:1000 and then added to an Invitrogen hsCRP (high-sensitivity CRP) human ELISA
kit (#KHA0032). An incremental CRP concentration standard range of 0–2.1 mg/L was used and
the ELISA plate was analyzed on a microplate reader at a wavelength of 490 nm.
Statistical analysis
BMI and ELISA hsCRP levels from the same individual were compared in a box plot graph
(Figure 1). Six individuals exceeded the 2.1 mg/L
maximum CRP value for the assay standard curve. These samples were included in the
statistical analyses using the value of 2.1 mg/L (a likely underestimate of their actual
circulating CRP levels). Supplemental questionnaire data collected did not indicate any
major health problems that might affect CRP levels (i.e., recent injury, illness, or
infection). To determine the anthropometric measurement most correlated with CRP level, a
Pearson correlation was performed between CRP and nine anthropometric measurements of body
fat (Table 1). A few individuals were missing
some measurements, so each comparison included 62–64 participants’ data. For a more
detailed view of the relationship between suprailiac skinfold thickness and CRP level, we
generated a fit plot with 95% confidence intervals (Figure 2). We then applied a stepwise
(bidirectional) regression analysis with a set entrance and stay level at 0.05 (Suppl.
Tables S3–S5).
Results
Women from each BMI category and ELISA hsCRP level from the same individual were compared
using a box plot graph (Figure 1). The average CRP
level for all 64 Ni-Vanuatu women was 0.97 mg/L. For the underweight BMI category (n=4), the
CRP average was 0.41 mg/L and only one sample exceeded 1.0 mg/L. The CRP average for the
average (n=27) and overweight (n=17) BMI categories were 0.89 mg/L and 0.91 mg/L,
respectively. However, the range of CRP levels for these two BMI categories were broad (0.52
mg/L to ≥2.1 mg/L). The obese BMI category (n=16) had the highest CRP average (1.2 mg/L)
with the majority of samples (n=12/16) being greater than the subclinical inflammation
threshold of 1.0 mg/L. However, six women in the obese category likely exceeded the assay
detection limit of 2.1 mg/L. Therefore, the actual means for CRP average in the overweight
and obese BMI categories is likely higher. The number of Ni-Vanuatu women from different
islands for each BMI category is also included. Individuals from Nguna and Efate, the
islands with the greatest market integration, comprise over half the overweight and obese
categories (n=18/33).
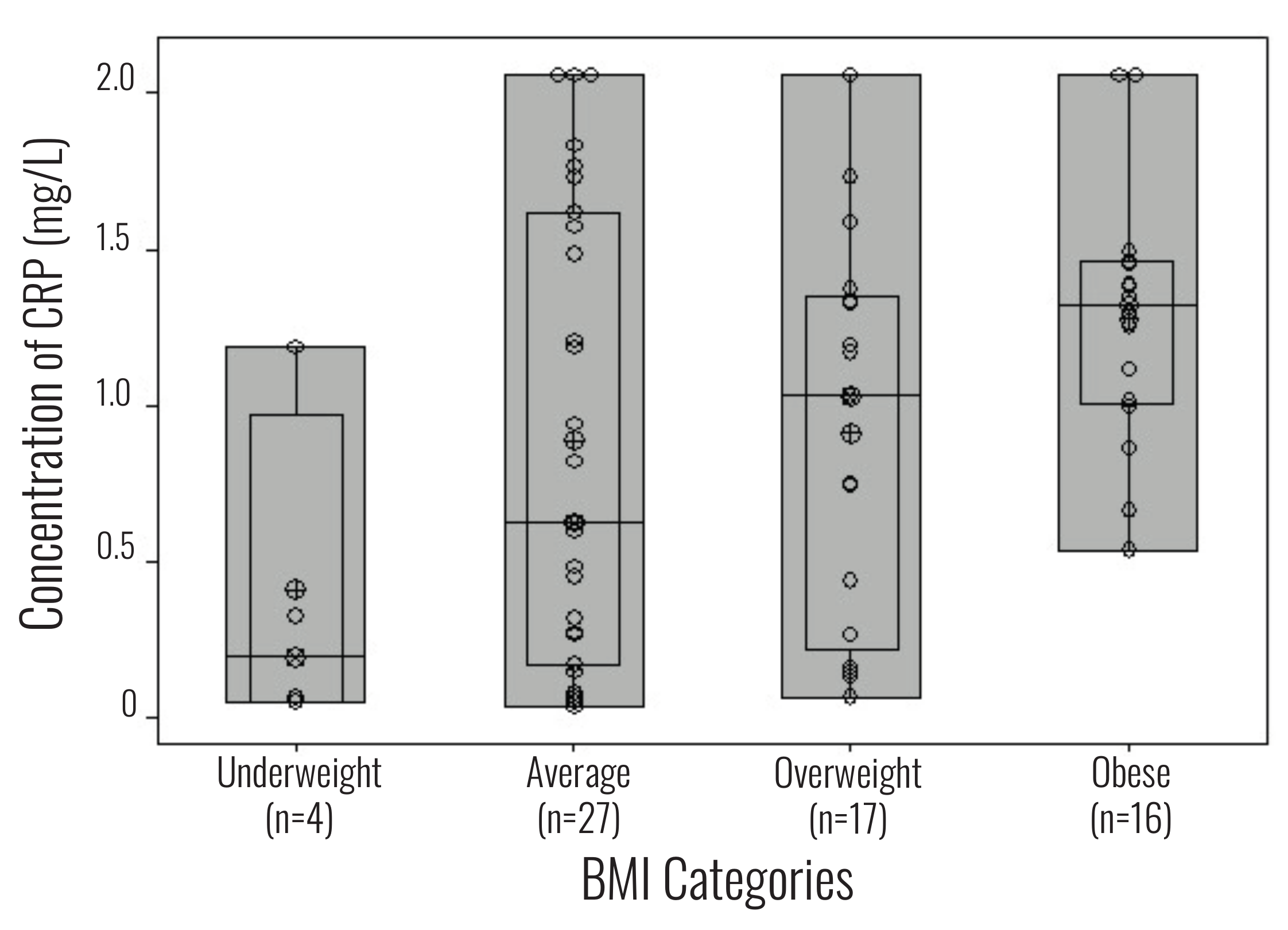
Figure 1 WHO BMI categories compared to CRP concentration (mg/L) from the same
individual.
Table 1 shows the Pearson correlations between the
nine anthropometrics and CRP. The anthropometric measurements that were significantly
correlated with CRP levels include BIA (p=0.257, r2=0.040), BMI (p=0.249,
r2=0.047), waist circumference (p=0.325, r2=0.009), and suprailiac
skinfold thickness (p=0.373, r2=0.003). Peripheral and upper body measures of fat
were not significantly correlated with CRP levels. The anthropometric measurements
correlated most with CRP level were the suprailiac skinfold (p=0.003) and waist
circumference (p=0.009). Based on the ANOVA analysis that resulted in that p-value of less
than 0.05, we proceeded with a stepwise regression (Suppl. Tables S3–S5). We observed that by
incorporating CRP as the input value, no variable was removed, and the fitted model was
reproducing the similar correlation as in the ANOVA analysis. The suprailiac skinfold was
the only anthropometric to enter the model and remain, explaining approximately 14%
(r2=0.139) of CRP variance. Figure 2
illustrates the regression trend line with 95% confidence intervals relating suprailiac
skinfold to CRP level.
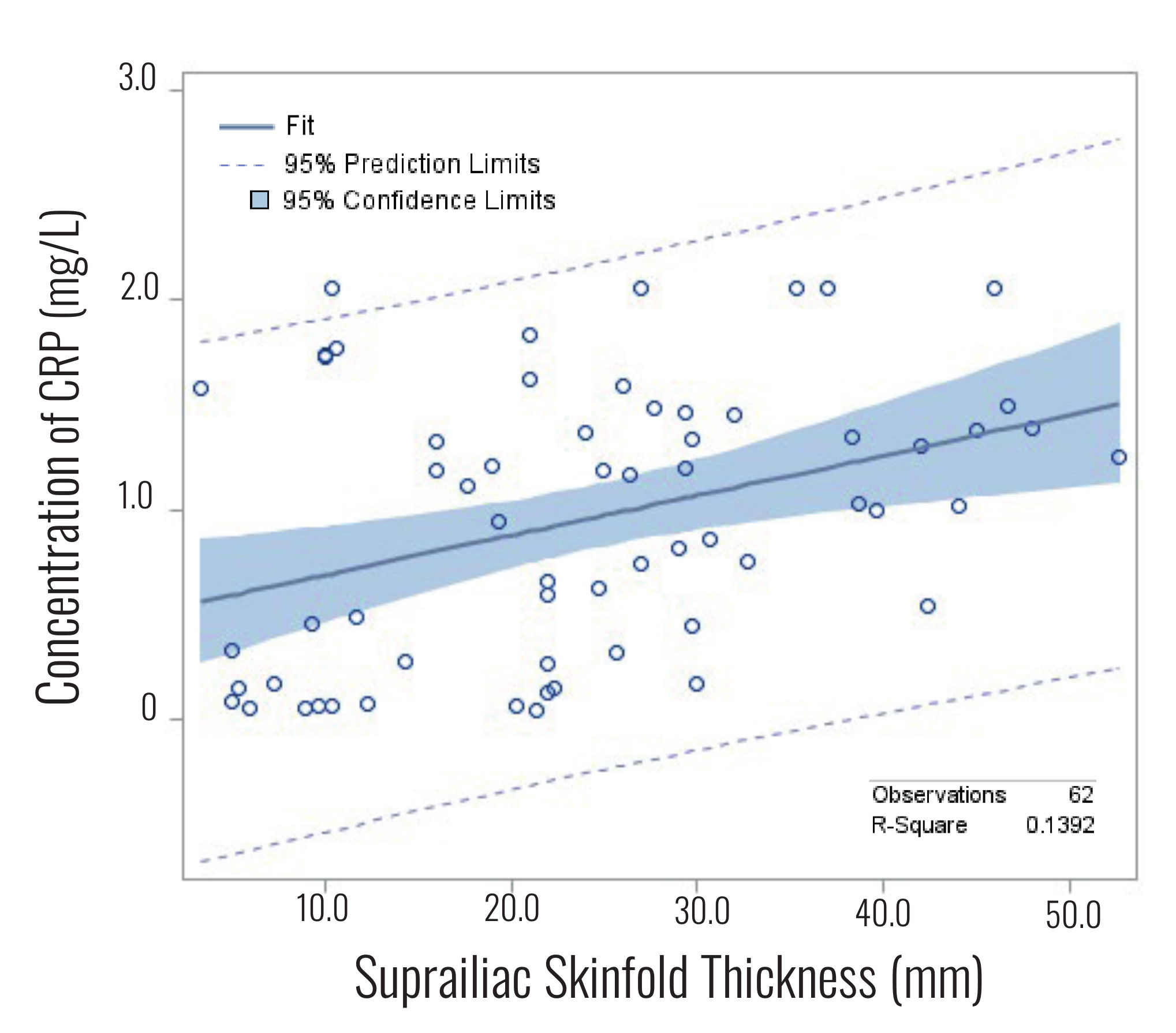
Figure 2 Fit plot for CRP level versus suprailiac skinfold thickness from the same
individual.
Discussion
Sociocultural changes resulting from colonization, resource exploitation and more recently,
global market integration have caused the world’s highest rates of obesity throughout the
Pacific Islands. In Vanuatu, the obesity rate is highest in the urban capital of Efate, but
obesity has also increased in peri-urban (e.g., Nguna) and rural islands (e.g., Aneityum)
due to growing tourism, greater access to non-traditional dietary items and infectious
disease control (Dancause et al. 2011). The increasing
risk for developing a NCD are therefore an alarming public health concern for Ni-Vanuatu.
The need for more accurate CVD risk assessments led to a re-consideration of BMI cut-off
action points for Asians and Pacific Islanders (WHO Expert
Consultation 2004). High-sensitivity CRP testing can improve the accuracy of CVD
risk assessments (Brooks et al. 2010; Danesh et al. 2000; Pearson et al. 2003); however, studies show that there are population differences
in baseline CRP level 2012)(e.g., Choi et al.. When
developing public health strategies for non-European populations, human biologists and other
health researchers should therefore be aware of how population diversity may affect
interpreting hsCRP data and corresponding NCD-risk data.
This study includes older Ni-Vanuatu women because they have a greater range of BMIs and
are disproportionately at risk for developing a NCD (Olszowy et al. 2015; Sites et al. 2002;
Visser et al. 1999). We first analyzed Ni-Vanuatu
CRP levels across the four the WHO BMI categories (Figure
1) recommended for international usage (World
Health Organization
2000a).
Our results indicate that the range of CRP levels for the average and overweight (n=44) BMI
categories are broad (0.52 mg/L to ≥2.1 mg/L). Moreover, the majority (n=12/16) of
Ni-Vanuatu women in the obese BMI category have low-grade inflammation (>1.0 mg/L).
Pacific Islanders have a predisposition for storing excess abdominal fat, so they are at a
higher risk for developing CVDs at lower BMIs (Dancause
et al. 2010; Forouhi et al. 2001; McKeigue et al. 1991; WHO Expert Consultation 2004). The revised WHO guidelines for Asian populations
(World Health Organization
2000b) suggest that if the BMI clinical action cutoff value for identifying overweight Asian
individuals is 23 kg/m2 (Pan and Yeh 2008; WHO Expert Consultation
2004), then obesity should be a public health concern if a significant portion of the population
has a BMI of >23 kg/m2. Some Asian countries (e.g., Thailand and Vietnam) have now
adapted this guideline to help identify more individuals at risk for noncommunicable
diseases (Jitnarin et al. 2011; Trinh et al. 2009) and Dancause et al. (2010) also advocated for
its usage in Vanuatu. For the current study, if WHO clinical action values are applied to
Ni-Vanuatu women, then nine women who currently fall within the average BMI category would
be re-categorized as overweight. CRP values for five of these women also indicate they have
subclinical inflammation, which puts them at greater risk for CVDs. Currently, the AHA has
defined standard health risk categories for hsCRP; however, there is uncertainty if AHA
hsCRP cutoff values for CVD risk can be accurately utilized for a diversity of populations
(Sung et al. 2014).
Anthropometric measurements that explicitly estimate abdominal fat tissue have been shown
to be better predictors of individual CRP levels and CVD risk than BMI (Ford et al. 2004; McDade et al. 2009; Peters et al. 2018).
This is likely because fat stored near the abdomen is more metabolically active and is known
to increase CRP levels (Brooks et al. 2010; Park et al. 2005). Our CRP correlation analysis showed
that peripheral and upper body measures (lower and upper arm, triceps, and subscapular) were
not significantly correlated with CRP levels. BMI and BIA were significantly correlated;
however, suprailiac skinfold thickness and waist circumference measurements were better
predictors of CRP levels (Table S3). BMI is an
indicator of body size and BIA (for this study) measures lower body fat to lean mass,
whereas suprailiac skinfold thickness and waist circumference are central abdomen measures.
Our results suggest that abdominal fat was the strongest mediator of CRP levels in
Ni-Vanuatu women, which supports our hypothesis. Further supporting this finding is the
observation that East Asians tend to have a lower correlation between BMI and CRP levels in
comparison to other populations (Choi et al. 2012;
Sung et al. 2014). Previously reported BMI-CRP
correlation values ranged from 0.13–0.39 for East Asian women and 0.32–0.6 for European and
African American women (Choi et al. 2012). The
BMI-CRP correlation value for Ni-Vanuatu women was 0.25, within the reported range for East
Asian women, but less than expected for European or African women. Furthermore, a recent
study that included 500,000 participants from the UK found that measures of central
adiposity were more strongly associated with myocardial infarction than BMI, especially
among women (Peters et al. 2018). A centrally
located pattern of body fat distribution is also likely more pathogenic in Ni-Vanuatu women,
so direct measures of central adiposity may be the most informative anthropometrics when
estimating CVD risk.
Compared to BMI and BIA, there are additional benefits to using suprailiac skinfold
thickness and waist circumference measurements in remote sampling locations, including ease
of calculation and minimal equipment requirements (i.e., calipers and a tape measure). In
this study, suprailiac skinfold thickness was the most significantly correlated
anthropometric with CRP, but waist circumference had a comparable value (Table 1). Suprailiac skinfold thickness may be
considered as an intrusive measure because it requires privacy, gender matched survey staff
and participants, and substantial training for reproducible data collection. Thus, waist
circumference may ultimately be the easiest, most meaningful anthropometric measurement for
researchers in the field to collect. The World Health
Organization (2000a) has recommended waist circumference values to help identify central obesity, which includes
≥94 cm for men and ≥80 cm for women. For Asian populations, ≥90 cm for men and ≥80 cm for
women have been suggested (An et al. 2013; World Health Organization
2000b).
However, a waist circumference value that is clinically significant for identifying an
increased risk for CVDs needs to be determined specifically for Ni-Vanuatu and other Pacific
Islander populations.
Lowering CRP levels can help reduce the risk for developing CVDs (Brooks et al. 2010).
Thus, identifying different physiological and environmental factors that increase CRP
levels can in turn be useful for clinical and public health interventions. So far, BMI has
been cited as being a large single contributor to individual CRP levels. For instance,
previous studies for European and Taiwanese populations found that BMI accounted for 15% and
11.9% of the total variance in CRP levels, respectively (Huang et al. 2013; Kathiresan et al.
2006). Suprailiac skinfold thickness remained in our stepwise model and explained
approximately 14% of CRP level variance (Tables
S3–S5). Suprailiac skinfold thickness is
therefore the greatest single contributor to CRP variance in our dataset. However, in
addition to excess central adiposity, environmental factors may also help explain Ni-Vanuatu
CRP level variance. For example, environmental factors associated with elevated CRP levels
include greater consumption of high-glycemic index foods and psychosocial stress (McDade et al. 2006; Neuhouser et al. 2012; Steptoe et al. 2007). These are
features typical of a more westernized diet and greater degree of market integration. Olszowy et al. (2015) included a larger sample of women from this study in Vanuatu. Their results
show that Nguna and Efate, the islands experiencing the greatest degree of market
integration, also had the highest rates of obesity among women (28.3% and 43.4%,
respectively). In our sample set, individuals from Nguna and Efate comprised over half the
overweight and obese BMI categories (Figure 1). These
islands are characterized by energy rich, high sodium and low nutrient, processed foods, as
well as decreased physical activity, and increased socioeconomic status (Dancause et al. 2010; Olszowy et al. 2015). Because market integration and cultural change leads to
increased adiposity in Ni-Vanuatu women (Olszowy et al.
2015), there is also a greater risk for elevated CRP levels. A larger sample from
different islands will be necessary to identify specific risk factors with the greatest
impact.
Uncharacterized hereditary factors could also be contributing to differences in baseline
CRP levels. One estimate suggests that serum CRP interindividual variability is 35–40%
heritable (Pankow et al. 2001). Several studies
have also found correlations between CRP polymorphisms and serum level in
different populations (e.g., Ghaffari et al. 2014;
Zacho et al. 2010).
For example, genetic contribution to CRP level variance for North American and Chinese
populations was found to be 1.4% and 2.4%, respectively (Kathiresan et al. 2006; Huang et al.
2013). As stated previously, Pacific Island populations are underrepresented in
widely used genomic datasets (e.g., 1000 Genomes and gnomAD) and medical genetics research,
so it is still unknown if Ni-Vanuatu will genetically resemble other Pacific Island
populations and/or if there is population-specific CRP gene variation that
is clinically relevant.
Finally, an optimistic note for improving health status in Vanuatu is that although it is a
lower-income country, there is minimal food insecurity. This is because all Ni-Vanuatu have
access to substantial arable land, possess traditional horticultural knowledge and food can
be grown all year. In addition to growing their own food, Ni-Vanuatu can also purchase
processed food on most islands. This is significant because a greater reliance on
traditional practices can help maintain a healthier lifestyle, whereas greater market
integration increases the risk for obesity (Dancause et al. 2011). Notably,
Vanuatu differs from most other low-income nations which are often lacking in a diversity of
dietary options and access to fresh food. Overconsumption of processed food can therefore be
viewed as a choice in Vanuatu and this awareness could be emphasized in local public health
programs.
Conclusions
Our results show that the suprailiac skinfold thickness and waist circumference, both
measures of central obesity, were the best anthropometric predictors of CRP levels in older
Ni-Vanuatu woman. These results are consistent with previous studies on Asian populations as
well as medical findings demonstrating that abdominal obesity is linked to CRP levels and
risk for CVDs. Although suprailiac skinfold thickness and waist circumference are both easy
to measure, waist circumference may ultimately be the most convenient to use in a field
setting. Analyzing blood spot samples for biomarkers has also been widely used by field
researchers to complement health-related studies. By utilizing this approach, our results
show that hsCRP testing can also determine which Ni-Vanuatu individuals are likely at
greater risk for CVD-related outcomes within the overweight and obese WHO categories. In
order to more accurately determine elevated CVD risk in Ni-Vanuatu, additional clinical and
behavioral studies will be necessary to determine clinically significant cutoff points for
measures of central adiposity and CRP levels. To examine the contribution of inherited
variation compared to environmental factors on circulating CRP levels, future studies can
also assess CRP gene variation from populations experiencing differential
market integration. Finally, the gradient of obesity prevalence is largely a reflection of
the degree of market integration and differential availability of processed foods among the
islands. However, because Vanuatu has low food insecurity and processed foods are consumed
largely by choice rather than need, public health education and other inexpensive,
non-biomedical interventions may be fruitful in Vanuatu.
Ethics
This project was approved by the Binghamton University Institutional Review Board (protocol
number #1578-10). We also obtained permissions from the Vanuatu Ministry of Health and prior
to sampling in each village, we received permission from local chiefs and the community at
large through public meetings. Informed consent was obtained from each study
participant.
Appendix
Supplementary Tables
Table S1 Distribution of participant age for each
island
|
n |
Mean |
Std. |
| All Islands |
64 |
51.6 |
11.1 |
| Ambae |
13 |
46.7 |
6.2 |
| Futuna |
13 |
51.8 |
9.2 |
| Aneityum |
13 |
52.6 |
14.6 |
| Nguna |
13 |
47.5 |
8.3 |
| Efate |
12 |
64.6 |
11.4 |
Table S2 WHO BMI categories with number of study participants from each
island
|
Underweight
(< 18.5 kg/m2) |
Average
(18.5–24.5 kg/m2) |
Overweight
(25.0–29.9 kg/m2) |
Obese
(≥30.0 kg/m2) |
| Island |
|
|
|
|
| Ambae |
2 |
7 |
3 |
1 |
| Futuna |
0 |
8 |
4 |
1 |
| Aneityum |
0 |
7 |
2 |
4 |
| Nguna |
1 |
2 |
6 |
4 |
| Efate |
1 |
3 |
2 |
6 |
| Total (n) |
4 |
27 |
17 |
16 |
Table S3 ANOVA for interactivity between CRP and other
anthropometrics
| Source |
DF |
Sum of
Squares |
Mean
Square |
F Value |
Pr > F |
r2 |
| Model* |
1 |
3508467 |
3508467 |
9.70 |
0.003 |
0.139 |
| Error |
60 |
21696263 |
361604 |
|
|
|
| Corrected
Total |
61 |
25204730 |
|
|
|
|
Table S4 Stepwise bidirectional regression analysis with anthropometric
input
| Step |
Variable Entered |
Variable Removed |
Partial R-square |
Model R-square |
F value |
Pr > F |
| 1 |
Suprailiac Sknflds |
(none) |
0.1392 |
0.1392 |
9.7 |
0.0028 |
Table S5 CRP regression model equationa
| Variable |
Parameter Estimate |
F Value |
Pr > F |
| Intercept |
4.9468792 |
8.85 |
0.0042 |
| Supra_Sknflds |
0.01910462 |
9.70 |
0.0014 |
Acknowledgements
We would like to acknowledge the Vanuatu Ministry of Health, the leadership of each
community we surveyed, and the many health workers and local assistants who aided us in data
collection for this project. We also thank the participants, without whose willingness to
participate, this project could not have been possible. This study was funded by the
following: Wenner-Gren Foundation for Anthropological Research; Binghamton University
Laboratory of Evolutionary Anthropology and Health; Harpur College Grants in Support of
Research, Scholarship and Creative Work.
References
Allen, M. R. (1968). The establishment of
Christianity and cash‐cropping in a New Hebridean
community. The Journal of Pacific History 3 (1), 25–46. https://doi.org/10.1080/00223346808572123.
An, Y./Yi, S./Fitzpatrick, A./Gupta, V./Prak, P.
R./Oum, S./LoGerfo, J. P. (2013). Appropriate body mass index and waist circumference
cutoff for overweight and central obesity among adults in Cambodia. PloS One 8 (10),
e77897. https://doi.org/10.1371/journal.pone.0077897.
Brooks, G. C./Blaha, M. J./Blumenthal, R.
S./Brooks, Gabriel C./Blaha, Michael J./Blumenthal, Roger S. (2010). Relation of
C-reactive protein to abdominal adiposity. The American Journal of Cardiology 106 (1),
56–61. https://doi.org/10.1016/j.amjcard.2010.02.017.
Central Intelligence Agency (2021). Obesity –
adult prevalence rate. The World Factbook, Langley (VA).
Choi, J./Joseph, L./Pilote, L. (2012). Obesity and
C‐reactive protein in various populations: a
systematic review and meta‐analysis. Obesity Reviews 14
(3), 232–244. https://doi.org/10.1111/obr.12003.
Dancause, K. N./DeHuff, C./Soloway, L. E./Vilar,
M./Chan, C./Wilson, M./Tarivonda, L./Regenvanu, R./Kaneko, A./Garruto, R. M./Lum, J. K.
(2011). Behavioral changes associated with economic development in the South Pacific:
health transition in Vanuatu. American Journal of Human Biology 23 (3), 366–376.
https://doi.org/10.1002/ajhb.21146.
Dancause, K. N./Vilar, M./DeHuff, C./Wilson,
M./Soloway, L. E./Chan, C./Lum, J. K./Garruto, R. M. (2010). Relationships between body
size and percent body fat among Melanesians in Vanuatu. Asia Pacific Journal of Clinical
Nutrition 19 (3), 425–431.
Dancause, K. N./Vilar, M./Wilson, M./Soloway, L.
E./DeHuff, C./Chan, C./Tarivonda, L./Regenvanu, R./Kaneko, A./Lum, J. K./Garruto, R. M.
(2013). Behavioral risk factors for obesity during health transition in Vanuatu, South
Pacific. Obesity 21 (1), E98‐E104. https://doi.org/10.1002/oby.20082.
Danesh, J./Whincup, P./Walker, M./Lennon,
L./Thomson, A./Appleby, P./Gallimore, J. R./Pepys, M. B. (2000). Low grade inflammation
and coronary heart disease: prospective study and updated meta-analyses. BMJ 321 (7255),
199–204. https://doi.org/10.1136/bmj.321.7255.199.
DeLoach, S./Falkner, B./Keith, S. W./Gidding, S.
S. (2014). Obesity associated inflammation in African American adolescents and adults.
The American Journal of the Medical Sciences 347 (5), 357–363. https://doi.org/10.1097/MAJ.0b013e31829555f0.
Ding, D./Wang, M./Su, D./Hong, C./Li, X./Yang,
Y./Zhang, Y./Hu, G./Ling, W. (2015). Body mass index, high-sensitivity C-reactive
protein and mortality in Chinese with coronary artery disease. PloS One 10 (8),
e0135713. https://doi.org/10.1371/journal.pone.0135713.
Doumatey, A. P./Lashley, K. S./Huang, H./Zhou,
J./Chen, G./Amoah, A./Agyenim‐Boateng, K./Oli,
J./Fasanmade, O./Adebamowo, C. A./Adeyemo, A. A./Rotimi, C. N. (2010). Relationships
among obesity, inflammation, and insulin resistance in African Americans and West
Africans. Obesity 18 (3), 598–603. https://doi.org/10.1038/oby.2009.322.
Ford, E. S./Giles, W. H./Mokdad, A. H./Myers, G.
L. (2004). Distribution and correlates of C-reactive protein concentrations among adult
US women. Clinical Chemistry 50 (3), 574–581. https://doi.org/10.1373/clinchem.2003.027359.
Forouhi, N. G./Sattar, N./McKeigue, P. M. (2001).
Relation of C-reactive protein to body fat distribution and features of the metabolic
syndrome in Europeans and South Asians. International Journal of Obesity 25 (9),
1327–1331. https://doi.org/10.1038/sj.ijo.0801723.
Ghaffari, M. A./Sede, S. A./Rashtchizadeh,
N./Mohammadzadeh, G./Majidi, S. (2014). Association of CRP gene polymorphism with CRP
levels and Coronary Artery Disease in Type 2 Diabetes in Ahvaz, southwest of Iran.
Bioimpacts 4 (3), 133. https://doi.org/10.15171/bi.2014.006.
Ghosh, C. (2003). Healthy people 2010 and Asian
Americans/Pacific islanders: defining a baseline of information. American Journal of
Public Health 93 (12), 2093–2098. https://doi.org/10.2105/ajph.93.12.2093.
Gill, T. (2006). Epidemiology and health impact of
obesity: an Asia Pacific perspective. Asia Pacific Journal of Clinical Nutrition 15,
Suppl:3–14.
Huang,
C.‐C./Chung,
C.‐M./Leu, H.‐B./Lin,
T.‐H./Hung,
S.‐l./Wu, T.‐C./Huang,
P.‐H./Lin,
S.‐J./Pan, W.‐H./Chen,
J.‐W. (2013). Genetic variation in C-reactive protein
in ethnic Chinese population in Taiwan. European Journal of Clinical Investigation 43
(5), 449–456. https://doi.org/10.1111/eci.12067.
Jeemon, P./Prabhakaran,
D./Ramakrishnan, L./Gupta, R./Ahmed, F./Thankappan, K. R./Kartha, C.
C./Chaturvedi, V./Reddy, K. S. (2011). Association of high sensitive C-reactive protein (hsCRP) with established cardiovascular
risk factors in the Indian population. Nutrition & Metabolism 8 (1), 1–8. https://doi.org/10.1186/1743-7075-8-19.
Jitnarin, N./Kosulwat, V./Rojroongwasinkul,
N./Boonpraderm, A./Haddock, C. K./Poston, W. S.C./Jitnarin, N./Poston, W. S. C. (2011).
Prevalence of overweight and obesity in Thai population: results of the National Thai
Food Consumption Survey. Eating and Weight Disorders 16 (4), e242–e249. https://doi.org/10.1007/BF03327467.
Kaneko, A./Taleo, G./Kalkoa, M./Yamar,
S./Kobayakawa, T./Björkman, A. (2000). Malaria eradication on islands.
Lancet 356 (9241), 1560–1564. https://doi.org/10.1016/S0140-6736(00)03127-5.
Kathiresan, S./Larson, M. G./Vasan, R. S./Guo,
C.-Y./Gona, P./Keaney Jr, J. F./Wilson, P. W. F./Newton-Cheh, C./Musone, S. L./Camargo,
A. L./Keaney, J. F./Drake, J. A./Levy, D./O'Donnell, C. J./Hirschhorn, J. N./Benjamin,
E. J. (2006). Contribution of clinical correlates and 13 C-reactive protein gene
polymorphisms to interindividual variability in serum C-reactive protein level.
Circulation 113 (11), 1415–1423. https://doi.org/10.1161/CIRCULATIONAHA.105.591271.
Kayser, M. (2010). The human genetic history of
Oceania: near and remote views of dispersal. Current Biology 20 (4), R194–R201.
https://doi.org/10.1016/j.cub.2009.12.004.
Khoo, C. M./Sairazi, S./Taslim, S./Gardner, D./Wu,
Y./Lee, J./van Dam, R. M./Tai, E. S. (2011). Ethnicity modifies the relationships of
insulin resistance, inflammation, and adiponectin with obesity in a multiethnic Asian
population. Diabetes Care 34 (5), 1120–1126. https://doi.org/10.2337/dc10-2097.
Klein, S./Allison, D. B./Heymsfield, S. B./Kelley,
D. E./Leibel, R. L./Nonas, C./Kahn, R. (2007). Waist circumference and cardiometabolic
risk: a consensus statement from shaping America’s health: Association for Weight
Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for
Nutrition; and the American Diabetes Association. The American Journal of Clinical
Nutrition 85 (5), 1197–1202. https://doi.org/10.1093/ajcn/85.5.1197.
Lim, S./Jang, H. C./Lee, H. K./Kimm, K. C./Park,
C./Cho, N. H. (2006). The relationship between body fat and C-reactive protein in
middle-aged Korean population. Atherosclerosis 184 (1), 171–177. https://doi.org/10.1016/j.atherosclerosis.2005.04.003.
Lipson, M./Skoglund, P./Spriggs, M./Valentin,
F./Bedford, S./Shing, R./Buckley, H./Phillip, I./Ward, G. K./Mallick, S./Rohland,
N./Broomandkhoshbacht, N./Cheronet, O./Ferry, M./Harper, T. K./Michel, M./Oppenheimer,
J./Sirak, K./Stewardson, K./Auckland, K./Hill, A. V. S./Maitland, K./Oppenheimer, S.
J./Parks, T./Robson, K./Williams, T. N./Kennett, D. J./Mentzer, A. J./Pinhasi, R./Reich,
D. (2018). Population turnover in remote Oceania shortly after initial settlement.
Current Biology 28 (7), 1157–1165. https://doi.org/10.1016/j.cub.2018.02.051.
Lohman, T. G./Roche, A. F./Martorell, R. (Eds.)
(1988). Anthropometric standardization reference manual. Champaign, Ill., Human Kinetics
Books.
Lum, J. K./Cann, R. L. (1998). mtDNA and language
support a common origin of Micronesians and Polynesians in Island Southeast Asia.
American Journal of Physical Anthropology 105 (2), 109–119. https://doi.org/10.1002/(SICI)1096-8644(199802)105:2<109::AID-AJPA1>3.3.CO;2-M.
MacClancy, J. (1981). To kill a bird with two
stones. A short history of Vanuatu. Port Vila, Vanuatu Cultural Centre.
McDade, T. W./Hawkley, L. C./Cacioppo, J. T.
(2006). Psychosocial and behavioral predictors of inflammation in middle-aged and older
adults: the Chicago health, aging, and social relations study. Psychosomatic Medicine 68
(3), 376–381. https://doi.org/10.1097/01.psy.0000221371.43607.64.
McDade, T. W./Rutherford, J. N./Adair, L./Kuzawa,
C. (2009). Population differences in associations between C-reactive protein
concentration and adiposity: comparison of young adults in the Philippines and the
United States. The American Journal of Clinical Nutrition 89 (4), 1237–1245. https://doi.org/10.3945/ajcn.2008.27080.
McKeigue, P. M./Shah, B./Marmot, M. G. (1991).
Relation of central obesity and insulin resistance with high diabetes prevalence and
cardiovascular risk in South Asians. Lancet 337 (8738), 382–386. https://doi.org/10.1016/0140-6736(91)91164-P.
McLennan, A. K./Ulijaszek, S. J. (2015). Obesity
emergence in the Pacific islands: why understanding colonial history and social change
is important. Public Health Nutrition 18 (8), 1499–1505. https://doi.org/10.1017/S136898001400175X.
NCD Risk Factor Collaboration (2019). Rising rural
body-mass index is the main driver of the global obesity epidemic in adults. Nature 569
(7755), 260. https://doi.org/10.1038/s41586-019-1171-x.
Neuhouser, M. L./Schwarz, Y./Wang, C./Breymeyer, K./Coronado, G./Wang, C. Y./Noar, K./Song, X./Lampe, J. W. (2012).
A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. Journal of Nutrition 142 (2), 369-374.
https://doi.org/10.3945/jn.111.149807.
Neovius, M./Hemmingsson, E./Freyschuss, B./Uddén,
J. (2006). Bioelectrical impedance underestimates total and truncal fatness in
abdominally obese women. Obesity 14 (10), 1731–1738. https://doi.org/10.1038/oby.2006.199.
Olszowy, K. M./Pomer, A./Dancause, K. N./Sun,
C./Silverman, H./Lee, G./Chan, C. W./Tarivonda, L./Regenvanu, R./Kaneko, A./Weitz, C.
A./Lum, J. K./Garruto, R. M. (2015). Impact of modernization on adult body composition
on five islands of varying economic development in Vanuatu. American Journal of Human
Biology 27 (6), 832–844. https://doi.org/10.1002/ajhb.22734.
Pan, W.-H./Yeh, W.-T. (2008). How to define
obesity? Evidence-based multiple action points for public awareness, screening, and
treatment: an extension of Asian-Pacific recommendations. Asia Pacific Journal of
Clinical Nutrition 17 (3), 370.
Pankow, J. S./Folsom, A. R./Cushman, M./Borecki,
I. B./Hopkins, P. N./Eckfeldt, J. H./Tracy, R. P. (2001). Familial and genetic
determinants of systemic markers of inflammation: the NHLBI family heart study.
Atherosclerosis 154 (3), 681–689. https://doi.org/10.1016/S0021-9150(00)00586-4.
Park, H. S./Park, J. Y./Yu, R. (2005).
Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α
and IL-6. Diabetes Research and Clinical Practice 69 (1), 29–35. https://doi.org/10.1016/j.diabres.2004.11.007.
Pearson, T. A./Mensah, G. A./Alexander, R.
W./Anderson, J. L./Cannon III, R. O./Criqui, M./Fadl, Y. Y./Fortmann, S. P./Hong,
Y./Myers, G. L./Cannon, R. O./Rifai, N./Smith, S. C./Taubert, K./Tracy, R. P./Vinicor,
F. (2003). Markers of inflammation and cardiovascular disease: application to clinical
and public health practice: a statement for healthcare professionals from the Centers
for Disease Control and Prevention and the American Heart Association. Circulation 107
(3), 499–511. https://doi.org/10.1161/01.CIR.0000052939.59093.45.
Peters, S. A. E./Bots, S. H./Woodward, M. (2018).
Sex differences in the association between measures of general and central adiposity and
the risk of myocardial infarction: results from the UK Biobank. Journal of the American
Heart Association 7 (5), e008507. https://doi.org/10.1161/JAHA.117.008507.
Popkin, B. M. (2004). The nutrition transition: an
overview of world patterns of change. Nutrition Reviews 62 (7, Suppl. Pt 2), S140–S143.
https://doi.org/10.1111/j.1753-4887.2004.tb00084.x.
Popkin, B. M./Adair, L. S./Ng, S. W. (2012).
Global nutrition transition and the pandemic of obesity in developing countries.
Nutrition Reviews 70 (1), 3–21. https://doi.org/10.1111/j.1753-4887.2011.00456.x.
Saito, M./Ishimitsu, T./Minami, J./Ono, H./Ohrui,
M./Matsuoka, H. (2003). Relations of plasma high-sensitivity C-reactive protein to
traditional cardiovascular risk factors. Atherosclerosis 167 (1), 73–79. https://doi.org/10.1016/S0021-9150(02)00380-5.
Schafer, M. H./Ferraro, K. F./Williams, S. R.
(2011). Low socioeconomic status and body mass index as risk factors for inflammation in
older adults: conjoint influence on C-reactive protein? Journals of Gerontology Series
A: Biomedical Sciences and Medical Sciences 66 (6), 667–673. https://doi.org/10.1093/gerona/glr009.
Sergi, G./Rui, M. de/Stubbs, B./Veronese,
N./Manzato, E. (2017). Measurement of lean body mass using bioelectrical impedance
analysis: a consideration of the pros and cons. Aging Clinical and Experimental Research
29 (4), 591–597. https://doi.org/10.1007/s40520-016-0622-6.
Shin, H.-R./Varghese, C. (2014). WHO Western
Pacific regional action plan for the prevention and control of NCDs (2014-2020).
Epidemiology and Health 36, e2014007. https://doi.org/10.4178/epih/e2014007.
Sites, C. K./Toth, M. J./Cushman, M./L’Hommedieu,
G. D./Tchernof, A./Tracy, R. P./Poehlman, E. T. (2002). Menopause-related differences in
inflammation markers and their relationship to body fat distribution and
insulin-stimulated glucose disposal. Fertility and Sterility 77 (1), 128–135. https://doi.org/10.1016/S0015-0282(01)02934-X.
Skoglund, P./Posth, C./Sirak, K./Spriggs,
M./Valentin, F./Bedford, S./Clark, G. R./Reepmeyer, C./Petchey, F./Fernandes, D./Fu,
Q./Harney, E./Lipson, M./Mallick, S./Novak, M./Rohland, N./Stewardson, K./Abdullah,
S./Cox, M. P./Friedlaender, F. R./Friedlaender, J. S./Kivisild, T./Koki, G./Kusuma,
P./Merriwether, D. A./Ricaut, F./Wee, J. T. S./Patterson, N./Krause, J./Pinhasi,
R./Reich, D. (2016). Genomic insights into the peopling of the Southwest Pacific. Nature
538 (7626), 510–513. https://doi.org/10.1038/nature19844.
Steptoe, A./Hamer, M./Chida, Y. (2007).
The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis.
Brain, Behavior, and Immunity 21 (7), 901-912. https://doi.org/10.1016/j.bbi.2007.03.011.
Sung, K.-C./Ryu, S./Chang, Y./Byrne, C. D./Kim, S.
H. (2014). C-reactive protein and risk of cardiovascular and all-cause mortality in 268
803 East Asians. European Heart Journal 35 (27), 1809–1816. https://doi.org/10.1093/eurheartj/ehu059.
Swinburn, B. A./Sacks, G./Hall, K. D./McPherson,
K./Finegood, D. T./Moodie, M. L./Gortmaker, S. L. (2011). The global obesity pandemic:
shaped by global drivers and local environments. Lancet 378 (9793), 804–814. https://doi.org/10.1016/S0140-6736(11)60813-1.
Trinh, O. T. H./Nguyen, N. D./Phongsavan,
P./Dibley, M. J./Bauman, A. E. (2009). Prevalence and risk factors with overweight and
obesity among Vietnamese adults: Caucasian and Asian cut-offs. Asia Pacific Journal of
Clinical Nutrition 18 (2), 226–233. Available online at https://search.informit.org/doi/10.3316/informit.683855345951353 (accessed
11/19/2021).
Vilar, M. G. (2010). Origins and post-settlement
gene flow among culturally and linguistically distinct Pacific populations. Doctoral
dissertation. [New York], Binghamton University.
Visser, M./Bouter, L. M./McQuillan, G. M./Wener,
M. H./Harris, T. B. (1999). Elevated C-reactive protein levels in overweight and obese
adults. JAMA 282 (22), 2131–2135. https://doi.org/10.1001/jama.282.22.2131.
WHO Expert Consultation (2004). Appropriate
body-mass index for Asian populations and its implications for policy and intervention
strategies. Lancet 363 (9403), 157–163. https://doi.org/10.1016/S0140-6736(03)15268-3.
World Health Organization (2000a). Obesity:
preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series 894, 1–253. PMID: 11234459.
World Health Organization. Regional Office for the Western Pacific (2000b).
The Asia-Pacific perspective: redefining obesity and its treatment. Sydney : Health Communications Australia.
https://apps.who.int/iris/handle/10665/206936 (accessed 12/22/2021).
Zacho, J./Tybjærg-Hansen, A./Nordestgaard, B.
G./Tybjaerg-Hansen, A. (2010). C-reactive protein and all-cause mortality: the
Copenhagen City Heart Study. European Heart Journal 31 (13), 1624–1632. https://doi.org/10.1093/eurheartj/ehq103.

